Abstract
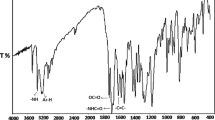
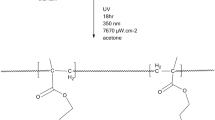
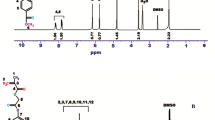
In this work, in a first step, a novel methacrylate monomer, 2-(4-hydroxyanilino)-2-oxoethyl-2-methylprop-2-enoate (HAOEME), was synthesized containing a phenolic hydroxyl group in the side branch. Then, the copolymers of this monomer were obtained by a free radical solution polymerization method with glycidyl methacrylate (GMA), a commercial monomer, at 65 °C in 1,4-dioxane solvent. The structural characterization of the synthesized monomers and copolymers was carried out using Fourier transform infrared (FTIR) spectroscopy, proton nuclear magnetic resonance (1H NMR), and 13C NMR techniques. Based on the monomer reactivity ratios obtained, the HAOEME monomer was found to be more reactive than GMA. The thermogravimetric analysis results showed that the thermal resistance of copolymers increased with an increase in the number of HAOEME units in the copolymer. It was also observed that the Tg value of copolymers increased with an increase of the molar fraction of HAOEMA units in the copolymer. The thermal degradation activation energy values of the polymers were determined using approaches including the Kissinger and Ozawa methods. The result of the biological activity studies revealed that the copolymers show moderate activity against different bacterial and fungal species. The swelling properties of the polymers were investigated using water. The variations in the swelling percentages were examined according to time and temperature parameters.














Similar content being viewed by others
References
Cironi A, Alvarez M, Albericio F (2006) Solid-phase chemistry in the total synthesis of non-peptidic natural products. Mini Rev Med Chem 6:11–25
Yakuphanoglu F, Erol I (2004) A novel organic semiconducting material: 2-(3-mesityl-3-methylcyclobutyl)-2-keto-ethyl methacrylate (MCKEMA). Physica B: Condensed Matter 352:378–382
Duncan R (2003) The dawning era of polymer therapeutics. Nat Rev Drug Discov 2:347–360
Erol I, Soykan C, Ahmedzade M (2002) Monomer reactivity ratios of the 2-(3-mesityl-3-methylcyclobutyl)-2-hydroxyethyl methacrylate and styrene system from 1H-NMR. J Polym Sci Part A-Polym Chem 40:1756–1763
Demirelli K, Coskun M, Erol I (2000) Copolymerization and monomer reactivity ratios of 2-(3-mesityl-3-methylcyclobutyl)-2-hydroxyethyl methacrylate with acrylonitrile. Eur Polym J 36:83–88
Erol I, Sarkaya S (2012) Copolymers of methacrylic and styrenic monomer based on the naphthalene: Synthesis, characterization, monomer reactivity ratios, and thermal properties. J Polym Res 19:9957
Soykan C, Erol I (2003) Synthesis, spectral and thermal properties of homo-and copolymers of 2-[(5-methylisoxazol-3-yl) amino]-2-oxo-ethyl methacrylate with styrene and methyl methacrylate and determination of monomer reactivity ratios. Eur Polym J 39:2261–2270
Kenawya E-R, IAbdel-Hay F, REl-Shanshoury A.E.-R., HEl-Newehy M, (1998) Biologically active polymers: synthesis and antimicrobial activity of modified glycidylmethacrylate polymers having a quaternary ammonium and phosphonium groups. J Control Release 50:145–152
Lang M, Jianzhong B, Wang S (1999) Synthesis and characterization of polycaprolactone / poly(ethylene oxide) / polylactide tri-component copolymers. J Biomater Sci Polym Ed 10:501–512
Martin C, Aibani N, Callan JF, Callan B (2015) Recent advances in amphiphilic polymers for simultaneous delivery of hydrophobic and hydrophilic drugs. Ther Deliv 7:15–31
Wang H, Chen QW, Zhou SQ (2018) Carbon-based hybrid nanogels: a synergistic nanoplatform for combined biosensing, bioimaging, and responsive drug delivery. Chem Soc Rev 47:4198–4232
Bicak TC, Soylemez S, Buber E, Toppare L, Yagci Y (2017) Poly( o- aminophenol) prepared by Cu(ıı) catalyzed air oxidation and its use a biosensing architecture. Polym Chem 8:3881–3888
Shi P, Zhang X, Liu Y, Duan Y, Li Y, Li Z, Han T (2020) A multi-stimuli-responsive AIE material switching among three emission states. Mater Lett 263:127214
Riess G (2003) Micellization of block copolymers. Prog Polym Sci 28:1107–1112
Moller S, Weisser J, Bischoff S, Schnabelrauch M (2007) Dextran and hyaluronan methacrylate based hydrogels as matrices for soft tissue reconstruction. Biomol Eng 24:496–504
Aydogdu Y, Erol I, Yakuphanoglu F et al (2003) Electrical conductivity and optical properties of copolymers based on 4-vinylpyridine and tetralincyclobutylhydroxyethylmethacrylate. Synth Met 139:327–334
Bhola R, Bhola SM, Liang H, Mishra B (2010) BBiocompatible denture polymers-a review. Trends Biomater Artif Organs 23:129–136
Hus S, Kolar M, Krajnc P (2016) Separation of heavy metals from water by functionalized glycidyl methacrylate poly (high internal phase emulsions). J Chromatogr A 1437:168–175
Reis AV, Fajardo AR, Schuquel ITA, Guilherme MR, Vidotti GJ, Rubira AF, Muniz EC (2009) Reaction of glycidyl methacrylate at the hydroxyl and carboxylic groups of poly(vinyl alcohol) and poly(acrylic acid): is this reaction mechanism still unclear? J Org Chem 74:3750–3757
Nasirtabrizi MH, Khodabandlou S, Zargin L, Parchehbaf Jadid A (2014) Modification of copolymers using nucleophilic reactions between glycidyl methacrylate and 9- anthracene carboxylic acid. Int J Ind Chem 5:6
Majer J, Krajnc P (2010) Amine functionalisations of glycidyl methacrylate based PolyHIPE monoliths. Macromol Symp 296:5–10
Ghosh S, Krishnamurti N (2000) Use of glycidyl methacrylate monomers for developing cross-linkable pressure sensitive adhesives. Eur Polym J 36:2125–2131
Yuan H, Yu B, Fan LH, Wang M, Zhu Y, Ding X, Xu F-J (2016) Multiple types of hydroxyl-rich cationic derivatives of PGMA for broad-spectrum antibacterial and antifouling coatings. Polym Chem 7:5709–5718
Guilherme MR, Reis AV, Takahashi SH, Rubira AF, Feitosa JPA, Muniz EC (2005) Synthesis of a novel superabsorbent hydrogel by copolymerization of acrylamide and cashew gum modified with glycidyl methacrylate. Carbohydr Chem 61:464–471
Jlassi K, Chandran S, Miˇcuˇsik M, Benna-Zayani M, Yagci Y, Thomas S, Chehimi MM (2015) Poly(glycidyl methacrylate)-grafted clay nanofiller for highly transparent and mechanically robust epoxy composites. Eur Polym J 72:89–101
Monzo A, Rejtar T, Guttman A (2009) Optimization of poly(GMA-co-EDMA) monolithic support for trypsin nanoreactor fabrication. J Chromatogr Sci 47:467–472
Naidu P.S.R, Norret M, Dunlop S.A, Fitzgerald M, Clemons T.D, Iyer K.S (2019) Novel hydrophilic copolymer-based nanoparticle enhances the therapeutic efficiency of doxorubicin in cultured MCF-7 cells: ACS Omega 4:17083–17089.
Arshady R, Ledwith A, (the late), (1981) G.W. Kenner, Phenolic resins for peptide-synthesis. 2. Reactivities of phenolic and amino functional-groups in styrene based resins. Macromol Chem Phy 182:41–46
Ham G (1989) “Copolymerization, High Polymers”: Interscience: New York 18
Shiomi T, Suzuki M, Tohyama M, Imai K (1989) Dependence of miscibility on copolymer composition for blends of poly(vinylchloride-co-vinyl acetate) and poly(n-butyl methacrylate-co-isobutyl methacrylate): Macromolecules 22:3578-3581
Bednarski R, Braun D, Borbély J, Kelen T (1990) Copolymerization of styrene and methyl alpha-Cyanocinnamate, 1. Synthesis, NMR-Assignment, reactıvity ratios. Makromol Chem 191:773–782
Chang TC, Wu KH, Chen HB, Ho SY, Chiu YS (1996) Thermal degradation of aged polytetrahydrofuran and its copolymers with 3-azidomethyl-3’-methyloxetane and 3-nitratomethyl-3’-methyloxetane by thermogravimetry. J Polym Sci Part A Polym Chem 34:3337–3343
Chang TC, Chen HB, Chiu YS, Ho SY (1997) Degradation of polydimethylsiloxane-block-polystyrene copolymer. Polym Degrad Stab 57:7–14
Miao L, Xu L, Narducy KW, Trudell ML (2009) First multigram preparation of SCP-123, a novel water-soluble analgesic. Org Process Res Dev 13:820–822
Fineman M, Ross SD (1950) Linear method for determining monomer reactivity ratios in copolymerization. J Polym Sci 5:259–262
Kelen T, Tudos F (1974) Analysis of the linear methods for determining copolymerization reactivity ratios. I. A new improved linear graphical method. J Mac Sci Part A-Chem 9:1–27
Connerton IF (1994) In Analysis of Membrane Proteins, Ed by Gould G.W.: Portland, London, 177
Chan ECZ, Pelczar MJ, Krieg NR (1993) Agar Diffusion Method, in Laboratory Exercies in Microbiology, ed by Chan, et all, Mc-Graw-Hill: New York, 225
Melville HW, Noble B, Watson WF (1949) Copolymerization. II. Molecular weight distribution and mean molecular weights in copolymerization systems. J Polym Sci 4:629–637
Cheng S, Zhang M, Dixit N, Moore RB, Long TE (2012) Nucleobase self-assembly in supramolecular adhesives. Macromolecules 45:805-812
Heinzmann C, Weder C, de Espinosa CLM (2016) Supramolecular polymer adhesives: advanced materials inspired by nature. Chem Soc Rev 45:342–358
Dinçer S, Köseli V, Kesim H, Rzaev ZMO, Pişkin E (2002) Radical copolymerization of N-isopropylacrylamide with anhydrides of maleic and citraconic acids. Eur Polym J 38:2143–2152
Ham G (1964) Copolymerization, High Polymers. Interscience, New York
Botta A, de Candia F, Palumbo R (1985) Glass transition in aliphatic polyamides. J Appl Polym Sci 30:1669–1677
Di Marzio EA, Gibbs JH (1963) Molecular interpretation of glass temperature depression by plasticizers. J Polym Sci A: Gen Papers 1:1417–1428
Fox Jr TG, Flory PJ (1950) Second order transition temperatures and related properties of polystyrene. I. Influence of molecular weight. J Appl Phys 21:581
Coskun M, Erol I, Coskun MF, Demirelli K (2002) Thermal degradation behaviour of two methacrylate polymers with side chain amide groups. Polym Degrad Stab 78:49–55
Coskun M, Demirelli K, Erol I, Ahmedzade M (1998) Thermal degradation of poly [2-(3-aryl-3-methylcyclobutyl)-2-hydroxyethyl methacrylate]. Polym Degrad Stab 61:493–497
Erol I, Soykan C, Ilter Z et al (2003) Thermal degradation of poly 2-[3-(6-tetralino)-3-methylcyclobutyl]-2-ketoethyl methacrylate. Polym Degrad Stab 81:287–295
Takeo O (1965) A new method of analyzing thermogravimetric data. Bull Chem Soc Jpn 38:1881–1886
Regnier N, Guibe C (1997) Methodology for multistage degradation of polyimide polymer. Polym Degrad Stab 55:165–172
Kissinger HE (1957) Reaction kinetics in differential thermal analysis. Anal Chem 29:1702-1706
Farhoosh R, Johnny S, Asnaashari M, Molaahmadibahraseman N, Sharif A (2016) Structure–AA relationships of o-hydroxyl, o-methoxy, and alkyl ester derivatives of p-hydroxybenzoic acid. Food Chem 194:128–134
Siquet C, Paiva-Martins F, Lima JLFC, Reis S, Borges F (2006) Antioxidant profile of dihydroxy- and trihydroxyphenolic acids–a structure-activity relationship study. Free Radical Res 40:433–442
Muñoz-Bonilla A, Fernández-García M (2012) Polymeric materials with antimicrobial activity. Prog Polym Sci 37:281–339
Lorenzo CA, Concheiro A, Dubovik AS, Grinberg NV, Burova TV, Grinberg VY (2005) Temperature-sensitive chitosan-poly(N-isopropylacrylamide) interpenetrated network with enhanced loading capacity and controlled release properties. J Control Release 102:629–641
Tasdelen B, Apohan NK, Guven O, Baysal BM (2004) Preparation of poly(N-isopropylacrylamide/itaconic acid) copolymeric hydrogels and their drug release behavior. Int J Pharm 278:343–351
Sen M, Yakar A (2001) Controlled release of antifungal drug terbinafine hydrochloride from poly(N-vinyl 2-pyrrolidone/itaconic acid) hydrogels. Int J Pharm 228:33–41
Acknowledgements
This study is supported by Afyon Kocatepe University Scientific Research Projects Coordination Unit. Project Number 14-FENB-26. The author is grateful to Dr. Zeki GÜRLER for the study of biological behavior.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Erol, İ., Özer, M. Copolymers of a novel amphiphilic methacrylate monomer based on the hydroxyl group: copolymerization kinetics, thermal properties, biological activity, and swelling behavior. J Polym Res 28, 372 (2021). https://doi.org/10.1007/s10965-021-02712-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-021-02712-w