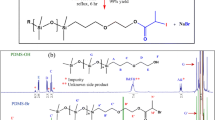
Abstract
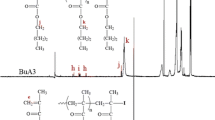
Homopolymerization of butadiene (Bu) in 1,4-dioxane (Dox) was performed at 70 °C using molecular iodine (I2) in the presence of 4,4’-azobis(4-cyanovaleric acid) (ACVA), resulting in α-carboxyl ω-iodine heterotelechelic polybutadiene. Effect of Dox concentration, molar ratio of ACVA to I2 and initiator type on the Bu conversion was studied. Inhibition of the reaction by molecular I2 was observed until complete consumption of the I2 (induction period). Then, polymerization initiated by carboxyl- functionalized alkyl iodides in situ generated during induction period. ACVA decomposition rate constant (kd), induction time \(\left({t}_{ind}\right)\) and \({k}_{p}^{2}/{k}_{t}\) ratio were calculated using Bu conversion as a function of time data. A good agreement between the theoretical and experimental changes in the conversion versus time was observed, indicating accuracy of the kinetic parameters estimated in this work. Experimental \({t}_{ind}\) was always less that theoretical one. It was attributed to reaction between Bu and I2, resulting in butadiene diiodide (I-Bu-I) compound. Formation of I-Bu-I species was further confirmed by 1H-NMR analysis of end functional groups (i.e. alkyl iodide and allyl iodide) of polybutadiene chains. Based on the 1H-NMR analysis, [I-Bu-I]/[I2]0 ratio was obtained for reaction B4 to be 0.235. It was found that among 1,2 and 1,4 additions, 1,2 addition of I2 to Bu is the dominant reaction. Exchange constant between the growing and dormant species (Cex) was estimated using GPC and conversion data to be in the range of 3.20–3.94. Then, \({M}_{n}\) and \(D\) evolution with conversion was investigated theoretically. Fraction of \(\alpha -\) carboxyl, \(\omega-\) iodide heterotelechelic PBu chains \(\left({f}_{HOOC-PBu-I}\right)\) relative to all chains was estimated from 1H-NMR data to be 87% for reaction B4.












Similar content being viewed by others
Data availability
Data will be made available on request.
References
Yu HS, Kim JS, Vasu V, Simpson CP, Asandei AD, Cu-Mediated Butadiene ATRP (2020) ACS Catal 10(12):6645–6663. https://doi.org/10.1021/acscatal.0c01207
Odian G (2004) Principles of Polymerization, p. 311, 4th ed., Wiley: New York, Chapter 3
Jang Y, Kwag G, Lee H (2000) High catalytic activity and stereoregularity of BF3 etherate cocatalyst of Nd-based catalyst in the polymerization of 1,3-butadiene. Polym J 32 (5), pp. 456–459. https://doi.org/10.1295/polymj.32.456
Iovu H, Hubca G, Simionescu E, Badea EG, Dimonie M (1997) Polymerization of butadiene and isoprene with the NdCl3 3TBP-TIBA catalyst system. Angew Makromol Chem 249:59–77. https://doi.org/10.1002/apmc.1997.052490105
Auad ML, Proi M, Borrajo J, Aranguren MI (2002) Rubber modified vinyl ester resins of different molecular weights. J Mater Sci 37 (19), art. no. 5102559, pp. 4117–4126. https://doi.org/10.1023/A:1020031701950
Nigam V, Setua DK, Mathur GN (2000) Chemical interaction and phase morphology development of carboxy terminated butadiene-co-acrylonitrile rubber toughened epoxy blends. Rubber Chem Technol 73 (5), pp. 830–838. https://doi.org/10.5254/1.3547622
Bouchal K, Žůrková E, Kálal J, Sufćák M, Seyček O (1980) Decomposition of hydrogen peroxide catalyzed by perchloric acid. Application of this initiation system in the preparation of HO‐terminated telechelic polymers of butadiene. Die Angewandte Makromolekulare Chemie, 86 (1), pp. 33–46. https://doi.org/10.1002/apmc.1980.050860103
Sadeghi GMM, Morshedian J, Barikani M (2003) The effect of initiator-to-monomer ratio on the properties of the polybutadiene-ol synthesized by free radical solution polymerization of 1,3-butadiene. Polym Int 52 (7), pp. 1083–1087. https://doi.org/10.1002/pi.1172
Zhang W, Zhang G, Du L, Zhang C, Li L, Zhu J, Pei J, Wu J (2018) Synthesis of hydroxyl-terminated polybutadiene bearing pendant carboxyl groups by combination of anionic polymerization and blue light photocatalytic thiol-ene reaction and its pH-triggered self-assemble behavior. React Funct Polym 127:161–167. https://doi.org/10.1016/j.reactfunctpolym.2018.04.003
Shipp DA (2011) Reversible-deactivation radical polymerizations. Polym Rev 51 (2), pp. 99–103. https://doi.org/10.1080/15583724.2011.566406
Ni Y, Zhang L, Cheng Z, Zhu X (2019) Iodine-mediated reversible-deactivation radical polymerization: A powerful strategy for polymer synthesis. Polym Chem 10 (20), pp. 2504–2515. https://doi.org/10.1039/c9py00091g
Hawker CJ, Bosman AW, Harth E (2001) New polymer synthesis by nitroxide mediated living radical polymerizations. Chem Rev 101 (12), pp. 3661–3688. https://doi.org/10.1021/cr990119u
Matyjaszewski K, Xia J (2001) Atom transfer radical polymerization. Chem Rev 101 (9), pp. 2921–2990. https://doi.org/10.1021/cr940534g. Kamigaito M, Ando T, Sawamoto M (2001) Metal-catalyzed living radical polymerization. Chem Rev 101 (12), pp. 3689–3745. https://doi.org/10.1021/cr9901182
Massoumi B, Abdollahi M, Fathi M, Entezami AA (2013) Hamidi, S. Synthesis of novel thermoresponsive micelles by graft copolymerization of N-isopropylacrylamide on poly(ε-caprolactone-co-α-bromo-ε- caprolactone) as macroinitiator via ATRP. J Polym Res 20 (2), art. no. 47. https://doi.org/10.1007/s10965-012-0047-7
Wang Z, Zhang Q, Zhan X, Chen F, Zhang X, Ouyang Q (2016) Precise preparation, microstructures, micromechanical behavior and morphology of well-defined PS-b-PS/Bd-b-PS triblock copolymers via RAFT seeded miniemulsion polymerization. J Polym Res 23 (12), art. no. 253. https://doi.org/10.1007/s10965-016-1152-9
Bar-Nes G, Hall R, Sharma V, Gaborieau M, Lucas D, Castignolles P, Gilbert RG (2009) Controlled/living radical polymerization of isoprene and butadiene in emulsion. Eur Polym J 45 (11), pp. 3149–3163. https://doi.org/10.1016/j.eurpolymj.2009.08.004
Iovu MC, Matyjaszewski K (2003) Controlled/living radical polymerization of vinyl acetate by degenerative transfer with alkyl iodides. Macromolecule 36 (25), pp. 9346–9354. https://doi.org/10.1021/ma034892+
Tonnar J, Pouget E, Lacroix-Desmazes P, Boutevin B (2008) Synthesis of poly(vinyl acetate)-b-poly(dimethylsiloxane)-b-poly(vinyl acetate) triblock copolymers by iodine transfer polymerization. Eur Polym J 44 (2), pp. 318–328. https://doi.org/10.1016/j.eurpolymj.2007.11.026
Miyajima T, Matsubara Y, Komatsu H, Miyamoto M, Suzuki K (2020) Development of a superabsorbent polymer using iodine transfer polymerization. Polym J 52 (4), pp. 365–373. https://doi.org/10.1038/s41428-019-0292-2
Goto A, Ohno K, Fukuda T (1998) Mechanism and kinetics of iodide-mediated polymerization of styrene. Macromolecules 31 (9), pp. 2809–2814. https://doi.org/10.1021/ma9712007
Lacroix-Desmazes P, Severac R, Boutevin B (2005) Reverse iodine transfer polymerization of methyl acrylate and n-butyl acrylate. Macromolecules 38 (15), pp. 6299–6309. https://doi.org/10.1021/ma050056j
Weerts PA, German AL, Gilbert RG (1991) Kinetic Aspects of the Emulsion Polymerization of Butadiene. Macromolecules 24 (7), pp. 1622–1628. https://doi.org/10.1021/ma00007a027
Mahdavian AR, Abdollahi M (2006) The comparison between initial charge, shot and modified shot processes and their effects on macrostructure of particles in emulsion copolymerization of styrene-butadiene-acrylic acid. React Funct Polym 66 (2), pp. 247–254. https://doi.org/10.1016/j.reactfunctpolym.2005.08.014
Kamachi M, Kajiwara A (1996) ESR study on radical polymerizations of diene compounds, determination of propagation rate constants. Macromolecules 29 (7), pp. 2378–2382. https://doi.org/10.1021/ma951280j
Brandrup J, Immergut EH, Grulke EA (1999) (eds.), Polymer Handbook, 4th ed., Wiley Inter Science: New Yourk
von Doering WE, Franck-Neumann M, Hasselmann D, Kaye RL (1972) On the Mechanism of a Diels-Alder Reaction. Butadiene and Its Dimers. J Am Chem Soc 94 (11), pp. 3833–3844. https://doi.org/10.1021/ja00766a029
Huybrechts G, Luyckx L, Vandenboom TH, Van Mele B (1977) Thermal dimerization of 1,3‐butadiene: Kinetics of the formation of cis, cis‐cycloocta‐1,5‐diene. Int J Chem Kinet 9 (2), pp. 283–293. https://doi.org/10.1002/kin.550090211
Li Y, Houk KN, (1993) Diels-Alder Dimerization of 1,3-Butadiene: An ab Initio CASSCF Study of the Concerted and Stepwise Mechanisms and Butadiene–Ethylene Revisited. J Am Chem Soc 115 (16), pp. 7478–7485. https://doi.org/10.1021/ja00069a055
Tasdelen MA, Kahveci MU, Yagci Y (2011) Telechelic polymers by living and controlled/living polymerization methods. Prog Polym Sci 36 (4), pp. 455–567. https://doi.org/10.1016/j.progpolymsci.2010.10.002
Mogaddam PN, Entezami A (2003) Synthesis of triblock copolymers of styrene and isoprene by a nitroxide-mediated living free radical polymerization. Chinese J Polym Sci (English Edition), 22 (1), pp. 55–61
Zhang X, Matyjaszewski K (1999) Synthesis of functional polystyrenes by atom transfer radical polymerization using protected and unprotected carboxylic acid initiators Macromolecules, 32 (22), pp. 7349–7353. https://doi.org/10.1021/ma990551d
Wei R, Luo Y, Li Z (2010) Synthesis of structured nanoparticles of styrene/butadiene block copolymers via RAFT seeded emulsion polymerization. Polymer 51 (17), pp. 3879–3886. https://doi.org/10.1016/j.polymer.2010.06.023
Yu Y, Zhang Q, Zhan X, Chen F (2013) Interfacially confined RAFT miniemulsion copolymerization of styrene and butadiene. J Appl Polym Sci 127 (4), pp. 2557–2565. https://doi.org/10.1002/app.37785
Ganjeh-Anzabi P, Haddadi-Asl V, Salami-Kalajahi M, Abdollahi M (2013) Kinetic investigation of the reversible addition-fragmentation chain transfer polymerization of 1,3-butadiene. J Polym Res 20 (9), art. no. 248. https://doi.org/10.1007/s10965-013-0248-8
Kaiser A, Brandau S, Klimpel M, Barner-Kowollik C (2010) Acrylonitrile-butadiene rubber (NBR) prepared via living/controlled radical polymerization (RAFT). Macromol Rapid Commun 31 (18), pp. 1616–1621. https://doi.org/10.1002/marc.201000162
Hlalele L, D'Hoog DR, Dürr CJ, Kaise A, Brandau S, Barner-Kowollik C (2014) RAFT-mediated ab initio emulsion copolymerization of 1,3-butadiene with acrylonitrile. Macromolecules 47 (9), pp. 2820–2829. https://doi.org/10.1021/ma500055q
Matyjaszewski K, Gaynor S, Wang JS (1995) Controlled Radical Polymerizations: The use of Alkyl Iodides in Degenerative Transfer. Macromolecules 28 (6), pp. 2093–2095. https://doi.org/10.1021/ma00110a050
Xu W, Zhan W, Li W, Yan J, Shen G, Li J (2012) Synthesis of poly(vinyl acetate) by degenerative transfer polymerization in the presence of iodine. J Appl Polym Sci 126 (1), pp. 104–109. https://doi.org/10.1002/app.36664
Abdollahi M, Bigdeli P, Hemmati M, Ghahramani M, Barari M (2015) Reverse iodine transfer polymerization of vinyl acetate and vinyl benzoate: Synthesis and characterization of homo- and copolymers. Polym Int 64 (12), pp. 1808–1819. https://doi.org/10.1002/pi.4985
Kim K, Ko NR, Rhee SE, Lee BH, Cho S (2012) Molecular control of polystyrene in the reverse iodine transfer polymerization (RITP)- Suspension process. Polymer 53 (19), pp. 4054–4059. https://doi.org/10.1016/j.polymer.2012.07.050
Moulay S (2014) Molecular iodine in monomer and polymer designing. Des Monomers Polym 17 (6), pp. 501–527. https://doi.org/10.1080/15685551.2013.867579
Taniyama T, Kurod T, Minam H, Okubo M (2012) Iodine transfer dispersion polymerization with CHI3 and reversible chain transfer-catalyzed dispersion polymerization with N-iodosuccinimide of methyl methacrylate in supercritical carbon dioxide. Polym J 44 (11), pp. 1082–1086. https://doi.org/10.1038/pj.2012.78
Lebreton P, Ameduri B, Boutevin B, Corpart JM, Juhue D (2000) Radical telomerization of 1,3-butadiene with perfluoroalkyl iodides. Macromol Chem Phys 201 (10), pp. 1016-1024. https://doi.org/10.1002/1521-3935(20000601)201:10<1016::AID-MACP1016>3.0.CO;2-H
Lebreton P, Ameduri B, Boutevin B, Cot D, Corpart JM (2002) Radical telomerization of 1,3-butadiene with perfluoroalkyl iodides in the presence of potassium carbonate. J Polym Sci, Part A: Polym Chem 40(21):3743–3756. https://doi.org/10.1002/pola.10470
Gu J, Yan X, Fu Z, Yang W, Sh Y (2013) Iodoform-mediated free radical emulsion polymerization of chloroprene. J Appl Polym Sci 128 (4), pp. 2291–2296. https://doi.org/10.1002/app.38082
Lacroix-Desmazes P, Tonnar J (2012) Degenerative Transfer with Alkyl Iodide, in: Polymer Science: A Comprehensive Reference, 1st Edition - Elsevier
Farrokhi M, Abdollahi M, Hemmati M (2014) Controlled radical copolymerization of vinyl acetate and dibutyl maleate by iodine transfer radical polymerization. Polym Int 63 (8), pp. 1494–1504. https://doi.org/10.1002/pi.4651
Germack DS, Wooley KL (2007) Isoprene polymerization via reversible addition fragmentation chain transfer polymerization. J Polym Sci, Part A: Polym Chem 45(17):4100–4108. https://doi.org/10.1002/pola.22226
Tonnar J, Lacroix-Desmazes P, Boutevin B (2006) Controlled radical Ab initio emulsion polymerization of n-butyl acrylate by Reverse Iodine Transfer Polymerization (RITP): Effect of the hydrolytic disproportionation of iodine. Macromol Rapid Commun 27 (20), pp. 1733–1738. https://doi.org/10.1002/marc.200600474
Hui J, Shi Y, Li T, Wu J, Fu Z (2015) Reverse iodine transfer polymerization (RITP) of chloroprene. RSC Advances 5 (55), pp. 44326–44335. https://doi.org/10.1039/c5ra04874e
Abdollahi M, Akbari HM (2021) Radical polymerization of butadiene mediated by molecular iodine: A comprehensive kinetic study on solution copolymerization with acrylonitrile. Polymer 214, art. no. 123255. https://doi.org/10.1016/j.polymer.2020.123255
Shin HC, Oh HG, Lee K, Lee BH, Choe S (2009) Emulsion polymerization of methyl methacrylate using the reverse iodine transfer polymerization (RITP) technique. Polymer 50 (18), pp. 4299–4307. https://doi.org/10.1016/j.polymer.2009.06.074
Reed SF (1971) Telechelic Diene Prepolymers II Carboxyl-Terminated Polydienes. J Poym Sci, Part A-1 Polym Chem 9:2147–2153
Reed Jr SF (1972) Telechelic diene prepolymers-3- Polymerization temperature study of polybutadienes. J Polym Sci Part A-1 Polym Chem 10 3 649 653
Farrokhi M, Abdollahi M, Rekabdar F, Hemmat M (2015) Reverse iodine transfer radical copolymerization of vinyl acetate and dibutyl maleate: synthesis and characterization of alternating and block copolymers. J Polym Res 22 (4), art. no. 43. https://doi.org/10.1007/s10965-015-0685-7
Tobolsky AV (1958) Dead-end Radical Polymerization. J Am Chem Soc 80 (22), pp. 5927–5929. https://doi.org/10.1021/ja01555a009
(a) Washington ID (2008) Dynamic Modelling of Emulsion Polymerization for the Continuous Production of Nitrile Rubber, MSc Thesis, University of Waterloo. (b) Washington ID, Duever TA, Penlidis A (2010) Mathematical modeling of acrylonitrile-butadiene emulsion copolymerization: Model development and validation. J Macromol Sci Part A: Pure Appl Chem 47 (8), pp. 747–769. https://doi.org/10.1080/10601325.2010.491436
Hovorka F, Schaefer RA, Dreisbach D (1936) The System Dioxane and Water. J Am Chem Soc 58 (11), pp. 2264–2267. https://doi.org/10.1021/ja01302a051
Kartavykh VP, Drach VA, Barantsevich YN, Abramenko YL (1977) Polymerization of diene hydrocarbons in the presence of azonitrile initiators containing carboxyl and hydroxyl groups. Polym Sci U.S.S.R., 19 (6), pp. 1413–1417. https://doi.org/10.1016/0032-3950(77)90271-4
Vernekar SP, Ghatge ND, Wadgaoknar PP (1988) Decomposition rate studies of azobisnitriles containing functional groups. J Polym Sci Part A: Polym Chem 26 (3), pp. 953–958. https://doi.org/10.1002/pola.1988.080260321
Moad G (2019) A Critical Assessment of the Kinetics and Mechanism of Initiation of Radical Polymerization with Commercially Available Dialkyldiazene Initiators. Prog Polym Sci 88:130–188. https://doi.org/10.1016/j.progpolymsci.2018.08.003
Moroni VAF (1967) Über den einfluß des lösungsmittels beim thermischen zerfall des azoisobuttersäuredinitrils. Die Makromolekulare Chemie 105:43–49. https://doi.org/10.1002/macp.1967.021050104
Bandermann F, Günther C, Schweer J (1996) Towards the propagation rate coefficient for the free-radical polymerization of 1,3-butadiene. Macromol Chem Phys 197 (3), pp. 1055–1069. https://doi.org/10.1002/macp.1996.021970324
Tutorskii IA, Sokolova LV (1977) Mechanism of the reaction of polybutadiene with molecular iodine. Polym Sci U.S.S.R., 19 (1), pp. 176–183. https://doi.org/10.1016/0032-3950(77)90164-2
Freed S, Sancier KM (1952) Experiments on Compound Formation in Solutions at Low Temperatures. Iodine and Olefins. J Am Chem Soc 74 (5), pp. 1273–1275. https://doi.org/10.1021/ja01125a039
Semsarzadeh MA, Abdollahi M (2008) Kinetic study of the free-radical polymerization of vinyl acetate in the presence of deuterated chloroform by 1H-NMR spectroscopy. J Appl Polym Sci 110 (3), pp. 1784–1796. https://doi.org/10.1002/app.28536
Szafko J, Feist W (1995) Solvation effect in thermal decomposition of 2,2′‐azoisobutyronitrile on the N,N‐dimethylformamide/methyl methacrylate system. J Polym Sci Part A: Polym Chem 33 (10), pp. 1637–1642. https://doi.org/10.1002/pola.1995.080331010
Szafko J, Feist W, Pabin-Szafko B (2000) Solvation effect in the thermal decomposition of 2,2′-azoisobutyronitrile in the three-component system. Journal of Polymer Science, Part A: Polym Chem 38 (12), pp. 2156-2166. https://doi.org/10.1002/(SICI)1099-0518(20000615)38:12<2156::AID-POLA40>3.0.CO;2-C
Netopilík M, Kratochvíl P (2003) Polystyrene-equivalent molecular weight versus true molecular weight in size-exclusion chromatography. Polymer 44 (12), pp. 3431–3436. https://doi.org/10.1016/S0032-3861(03)00258-1
Davis TP, O'Driscoll KF, Piton MC, Winnik MA (1991) Copolymerization propagation kinetics of styrene with alkyl acrylates. Polym Int 24 (2), pp. 65–70. https://doi.org/10.1002/pi.4990240202
Mark-Houwink Parameters for Polymers, by: American Polymer Standards Corporation, http://www.ampolymer.com/Mark-Houwink.html
Mayo FR (1943) Chain Transfer in the Polymerization of Styrene: The Reaction of Solvents with Free Radicals. J Am Chem Soc 65 (12), pp. 2324–2329. https://doi.org/10.1021/ja01252a021
Müller AHE, Zhuang R, Yan D, Litvinenko G (1995) Kinetic Analysis of “Living” Polymerization Processes Exhibiting Slow Equilibria. 1. Degenerative Transfer (Direct Activity Exchange between Active and “Dormant” Species). Application to Group Transfer Polymerization. Macromolecules 28 (12), pp. 4326–4333. https://doi.org/10.1021/ma00116a040
Acknowledgements
The author, M. Abdollahi, would like to acknowledge the Iran National Science Foundation (INSF) (grant number # 96010037) and Tarbiat Modares University, Tehran, Iran, for the financial support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Abdollahi, M., Akbari Hajiataloo, M. Radical polymerization of butadiene mediated by molecular iodine: a kinetic study of solution homopolymerization. J Polym Res 28, 311 (2021). https://doi.org/10.1007/s10965-021-02617-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-021-02617-8