Abstract
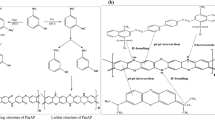
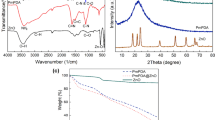
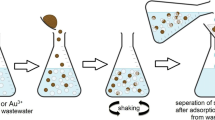
A polyazomethine poly-2,2’-hexamethylenebis(oxybenzaldehyde)-1,5-naphthalenediimine (PoHOBND) was prepared by polycondensation reaction of dialdehyde and 1,5-naphthlenediamine. The polymer was characterized through elemental microanalysis, FT-IR, 1HNMR, SEM, UV–Visible spectroscopy, fluorescence and thermal analysis. The polyazomethine (PoHOBND) showed violet colored fluorescence emissions and its thermal stability was up to 630˚C. The antimicrobial activities of the polyazomethine (PoHOBND) were also studied. An effective method was produced and employed at optimum conditions in remediation of Ni (II), Cu (II) and Co (II) from sewage wastewater samples and polyazomethine (PoHOBND) was used as sorbent. The sorption factors (adsorbate concentration, sorbent amount, shaking duration and pH) were optimized through multi-variant technique by using factorial design based on eighteen batch experiments. The metal ions present in wastewater were determined through AAS. The polyazomethine (PoHOBND) removed up to 77% Ni (II), 98% Cu (II) and 72% Co (II) from wastewater with RSD within 0.26–3.62%. The adsorption of Ni (II), Cu (II), and Co (II) on polyazomethine surface was confirmed through SEM images and EDX analysis. Kinetics and equilibrium of the adsorption were also examined. The sorption process of Ni (II), Cu (II), and Co (II) obeyed Langmuir isotherm with adsorption capacities of 25.64, 15.38 and 31.25 mg g−1 respectively and their kinetic results best suited to pseudo-second-order.





Similar content being viewed by others
References
Kumar A, Balouch A, Pathan AA, Mahar AM, Abdullah JMS, Mustafai FA, Zubair M, Laghari B, Panah P (2017) Remediation techniques applied for aqueous system contaminated by toxic Chromium and Nickel ion. Geology, Ecology, and Landscapes 1(2):143–153. https://doi.org/10.1080/24749508.2017.1332860
Wang S (2006) Cobalt—its recovery, recycling, and application. Jom 58(10):47–50. https://doi.org/10.1007/s11837-006-0201-y
Elshkaki A, Graedel T, Ciacci L, Reck BK (2016) Copper demand, supply, and associated energy use to 2050. Glob Environ Chang 39:305–315. https://doi.org/10.1016/j.gloenvcha.2016.06.006
Rathor G, Chopra N, Adhikari T (2014) Nickel as a pollutant and its management. Int Res J Environ Sci 3:94–98
Beyene HD, Berhe GB (2015) The level of heavy metals in portable water in Dowhan, Erop Wereda, Tigray. Ethiopia Journal of Natural Sciences Research 5(3):190–194
Khan S, Shahnaz M, Jehan N, Rehman S, Shah MT, Din I (2013) Drinking water quality and human health risk in Charsadda district, Pakistan. J Clean Prod 60:93–101. https://doi.org/10.1016/j.jclepro.2012.02.016
Kumar GP, Malla KA, Yerra B, Rao KS (2019) Removal of Cu (II) using three low-cost adsorbents and prediction of adsorption using artificial neural networks. Appl Water Sci 9(3):44. https://doi.org/10.1007/s13201-019-0924-x
Zietz BP, Dieter HH, Lakomek M, Schneider H, Keßler-Gaedtke B, Dunkelberg H (2003) Epidemiological investigation on chronic copper toxicity to children exposed via the public drinking water supply. Sci Total Environ 302(1–3):127–144. https://doi.org/10.1016/S0048-9697(02)00399-6
Araya M, Olivares M, Pizarro F, Llanos A, Figueroa G, Uauy R (2004) Community-based randomized double-blind study of gastrointestinal effects and copper exposure in drinking water. Environ Health Perspect 112(10):1068–1073. https://doi.org/10.1289/ehp.6913
Zhang X, Shi X, Ma L, Pang X, Li L (2019) Preparation of Chitosan Stacking Membranes for Adsorption of Copper Ions. Polymers 11(9):1463. https://doi.org/10.3390/polym11091463
Buxton S, Garman E, Heim KE, Lyons-Darden T, Schlekat CE, Taylor MD, Oller AR (2019) Concise review of nickel human health toxicology and ecotoxicology. Inorganics 7(7):89. https://doi.org/10.3390/inorganics7070089
Das K, Das S, Dhundasi S (2008) Nickel, its adverse health effects & oxidative stress. Indian J Med Res 128(4):412
Manohar D, Noeline B, Anirudhan T (2006) Adsorption performance of Al-pillared bentonite clay for the removal of cobalt (II) from aqueous phase. Appl Clay Sci 31(3–4):194–206. https://doi.org/10.1016/j.clay.2005.08.008
Bhatnagar A, Minocha A, Sillanpää M (2010) Adsorptive removal of cobalt from aqueous solution by utilizing lemon peel as biosorbent. Biochem Eng J 48(2):181–186. https://doi.org/10.1016/j.bej.2009.10.005
O’Connell DW, Birkinshaw C, O’Dwyer TF (2008) Heavy metal adsorbents prepared from the modification of cellulose: A review. Biores Technol 99(15):6709–6724. https://doi.org/10.1016/j.biortech.2008.01.036
Zeng X, Yu T, Wang P, Yuan R, Wen Q, Fan Y, Wang C, Shi R (2010) Preparation and characterization of polar polymeric adsorbents with high surface area for the removal of phenol from water. J Hazard Mater 177(1–3):773–780. https://doi.org/10.1016/j.jhazmat.2009.12.100
Kaya İ, Yıldırım M, Avcı A (2010) Synthesis and characterization of fluorescent polyphenol species derived from methyl substituted aminopyridine based Schiff bases: the effect of substituent position on optical, electrical, electrochemical, and fluorescence properties. Synth Met 160(9–10):911–920. https://doi.org/10.1016/j.synthmet.2010.01.044
Zaltariov MF, Cazacu M, Racles C, Musteata V, Vlad A, Airinei A (2015) Metallopolymers based on a polyazomethine ligand containing rigid oxadiazole and flexible tetramethyldisiloxane units. J Appl Polym Sci 132 (11). https://doi.org/10.1002/app.41631
Kim HJ, Lee JH, Lee M, Lee TS (2008) Optical switching and anion-induced chromogenic application in conjugated polyazomethine derivatives. React Funct Polym 68(12):1696–1703. https://doi.org/10.1016/j.reactfunctpolym.2008.09.010
Zaltariov M-F, Cazacu M, Shova S, Varganici C-D, Vacareanu L, Musteata V, Airinei A (2014) A silicon-containing polyazomethine and derived metal complexes: synthesis, characterization, and evaluation of the properties. Des Monomers Polym 17(7):668–683. https://doi.org/10.1080/15685551.2014.907623
Iwan A, Sek D (2008) Processible polyazomethines and polyketanils: from aerospace to light-emitting diodes and other advanced applications. Prog Polym Sci 33(3):289–345. https://doi.org/10.1016/j.progpolymsci.2007.09.005
Abd El-Lateef HM, Sayed AR, Shalabi K (2021) Synthesis and theoretical studies of novel conjugated polyazomethines and their application as efficient inhibitors for C1018 steel pickling corrosion behavior. Surfaces and Interfaces 23:101037. https://doi.org/10.1016/j.surfin.2021.101037
Moradi O, Mirza B, Norouzi M, Fakhri A (2012) Removal of Co (II), Cu (II) and Pb (II) ions by polymer based 2-hydroxyethyl methacrylate: thermodynamics and desorption studies. Iranian journal of environmental health science & engineering 9(1):31
Uğuzdoğan E, Denkbaş EB, Kabasakal OS (2010) The use of polyethyleneglycolmethacrylate-co-vinylimidazole (PEGMA-co-VI) microspheres for the removal of nickel (II) and chromium (VI) ions. J Hazard Mater 177(1–3):119–125. https://doi.org/10.1016/j.jhazmat.2009.12.004
Qureshi F, Memon SQ, Khuhawar MY, Jahangir TM (2021) Removal of Co2+, Cu2+ and Au3+ ions from contaminated wastewater by using new fluorescent and antibacterial polymer as sorbent. Polym Bull. 78:1505-1533. https://doi.org/10.1007/s00289-020-03170-y
Khuhawar M, Shah A, Shah AA (2009) Synthesis characterization and metal uptake study of Cu and Cd on poly-5, 5'-methylene-bis-(2-hydroxy benzaldehyde) ethylenediimine. J Chem Soc Pak 31(6):900–906. https://inis.iaea.org/search/search.aspx?orig_q=RN:40099923
Samal S, Das R, Sahoo D, Acharya S, Panda R, Rout R (1996) Chelating resins. III. Synthesis, characterization, and capacity studies of formaldehyde‐condensed phenolic schiff bases derived from 1, 2‐diamines and hydroxybenzaldehydes. J Appl Polym Sci 62(9):1437–1444. https://onlinelibrary.wiley.com/doi/10.1002/(SICI)1097-4628(19961128)62:9%3C1437::AID-APP13%3E3.0.CO;2-W
Samal S, Das R, Acharya S, Mohapatra P, Dey R (2002) A comparative study on metal ion uptake behavior of chelating resins derived from the formaldehyde-condensed phenolic Schiff bases of 4, 4′-diaminodiphenylsulfone and hydroxybenzaldehydes. Polym-Plast Technol Eng 41(2):229–246. https://doi.org/10.1081/PPT-120002565
Samal S, Das R, Dey R, Acharya S (2000) Chelating resins VI: Chelating resins of formaldehyde condensed phenolic Schiff bases derived from 4, 4′-diaminodiphenyl ether with hydroxybenzaldehydes—synthesis, characterization, and metal ion adsorption studies. J Appl Polym Sci 77(5):967–981. https://onlinelibrary.wiley.com/doi/10.1002/1097-4628(20000801)77:5%3C967::AID-APP3%3E3.0.CO;2-5
Pettit RK, Weber CA, Kean MJ, Hoffmann H, Pettit GR, Tan R, Franks KS, Horton ML (2005) Microplate Alamar blue assay for Staphylococcus epidermidis biofilm susceptibility testing. Antimicrob Agents Chemother 49(7):2612–2617. https://doi.org/10.1128/AAC.49.7.2612-2617.2005
Qureshi F, Khuhawar MY, Jahangir TM, Channar AH (2020) Synthesis and characterization of new thermally stable, antimicrobial and red-light-emitting poly(azomethine-ester)s. Polym Bull. https://doi.org/10.1007/s00289-020-03357-3
Catanescu O, Grigoras M, Colotin G, Dobreanu A, Hurduc N, Simionescu CI (2001) Synthesis and characterization of some aliphatic–aromatic poly(Schiff base)s. Eur Polymer J 37(11):2213–2216. https://doi.org/10.1016/S0014-3057(01)00119-7
İlhan S, Temel H, Sunkur M, Teğin İ (2008) Synthesis, structural characterization of new macrocyclic Schiff base derived from 1, 6-bis (2-formylphenyl) hexane and 2, 6-diaminopyridine and its metal complexes. Indian J Chem - Sect A Inorganic, Phys Theor Anal Chem 47:560–564
Qureshi F, Khuhawar MY, Jahangir TM (2018) Synthesis and Characterization of New Photo-responsive, Ortho and Para Oriented Azomethine Polymers. Acta Chimica Slovenica 65(3):718–729. https://doi.org/10.17344/acsi.2018.4419
Qureshi F, Khuhawar MY, Jahangir TM (2019) New Fluorescent, Thermally Stable and Film Forming Polyimines Containing Naphthyl Rings. Acta Chimica Slovenica 66(4):899–912. https://doi.org/10.17344/acsi.2019.5100
Qureshi F, Khuhawar MY, Jahangir TM, Channar AH (2016) Synthesis, characterization and biological studies of new linear thermally stable Schiff base polymers with flexible spacers. Acta Chimica Slovenica 63(1):113–120. https://doi.org/10.17344/acsi.2015.1994
Hasan S, Srivastava P, Talat M (2009) Biosorption of Pb (II) from water using biomass of Aeromonas hydrophila: central composite design for optimization of process variables. J Hazard Mater 168(2–3):1155–1162. https://doi.org/10.1016/j.jhazmat.2009.02.142
Mouelhi M, Marzouk I, Hamrouni B (2016) Optimization studies for water defluoridation by adsorption: application of a design of experiments. Desalin Water Treat 57(21):9889–9899. https://doi.org/10.1080/19443994.2015.1032363
Yasin Y, Mohamad M, Ahmad FB (2013) The application of response surface methodology for lead ion removal from aqueous solution using intercalated tartrate-Mg-Al layered double hydroxides Int J Chem Eng 2013 https://doi.org/10.1155/2013/937675
Ho Y-S (2006) Second-order kinetic model for the sorption of cadmium onto tree fern: a comparison of linear and non-linear methods. Water Res 40(1):119–125. https://doi.org/10.1016/j.watres.2005.10.040
Weber WJ, Morris JC (1963) Kinetics of adsorption on carbon from solution. J Sanit Eng Div 89(2):31–60
Yakout S, Elsherif E (2010) Batch kinetics, isotherm and thermodynamic studies of adsorption of strontium from aqueous solutions onto low cost rice-straw based carbons. Carbon-Sci Tech 1:144–153
Al-Shahrani H, Alakhras F, Al-Abbad E, Al-Mazaideh G, Hosseini-Bandegharaei A, Ouerfelli N (2018) Sorption of Cobalt (II) Ions from Aqueous Solutions Using Chemically Modified Chitosan. Glob Nest J 20(3):620–627. https://doi.org/10.30955/gnj.002804
Danjani A, Salisu A, Usman A (2016) Preparation and characterization of dialdehyde 2, 3-diaminopyridine starch chelating polymer and its sorption potential for Cd (II), Cu (II) and Ni (II) ions in aqueous media. Bayero Journal of Pure and Applied Sciences 9(2):174–178. https://doi.org/10.4314/bajopas.v9i2.32
Zalloum HM, Al-Qodah Z, Mubarak MS (2008) Copper Adsorption on Chitosan-Derived Schiff Bases. Journal of Macromolecular Science, Part A 46(1):46–57. https://doi.org/10.1080/10601320802515225
Acknowledgements
We acknowledge IARSCS, University of Sindh for giving us the opportunities to carry out this research work.
Funding
The authors declare that no external funding was available for this research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
We do not have any conflict of interest with any individual or organization. The work also does not involve any ethical conflicts.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Qureshi, F., Memon, S.Q., Khuhawar, M.Y. et al. Synthesis and application of fluorescent and thermally stable polyazomethine as adsorbent in the remediation of Ni (II), Cu (II) and Co (II) from wastewater systems. J Polym Res 28, 259 (2021). https://doi.org/10.1007/s10965-021-02582-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-021-02582-2