Abstract
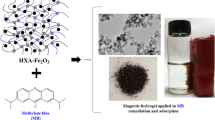
Several casein hydrogels were synthesized using glutaraldehyde as a crosslinker. The hydrogel prepared from 10 wt% casein and 5 wt% glutaraldehyde at pH = 7.5 showed the best adsorption performance for Cu2+ and methylene blue (MB). The morphology and thermal stability of the hydrogels were characterized by Fourier transform infrared spectrophotometry (FTIR), field emission scanning electron microscopy (FESEM) and thermo gravimetric analysis (TGA). Adsorption data were observed to fit well to pseudo second-order kinetics and the Freundlich-Langmuir switch model. The thermodynamic parameters of the adsorption showed that the adsorption of Cu2+ and MB into casein hydrogel was a spontaneous process.













Similar content being viewed by others
References
Carmalin Sophia A, Lima EC (2018) Removal of emerging contaminants from the environment by adsorption. Ecotoxicol Environ Saf 150:1–17
GilPavas E, Dobrosz-Gomez I, Gomez-Garcia MA (2019) Optimization and toxicity assessment of a combined electrocoagulation, H2O2/Fe2+/UV and activated carbon adsorption for textile wastewater treatment. Sci Total Environ 651:551-560
Senthilkumar K, Devi VC, Mothil S (2018) Adsorption studies on treatment of textile wastewater using low-cost adsorbent. Desalin Water Treat 123:90-100
Ni N, Zhang D, Dumont MJ (2018) Synthesis and characterization of zein-based superabsorbent hydrogels and their potential as heavy metal ion chelators. Polymer Bulletin (1):31–45
Shi W, Dumont MJ, Ly EB (2014) Synthesis and properties of canola protein-based superabsorbent hydrogels 54:172–180
Wattie B, Dumont MJ, Lefsrud M (2018) Synthesis and Properties of Feather Keratin-Based Superabsorbent Hydrogels. Waste Biomass Valoriz 9:391–400
Li N, Fu C, Zhang L (2014) Using casein and oxidized hyaluronic acid to form biocompatible composite hydrogels for controlled drug release. Mater Sci Eng C36:287–293
Fox PF, Mulvihill DM, Harris P (1990) Food gels, Elsevier Applied Science, London
AJE F, Teller SS, Jha AK, Jiao T, Hule RA, Clifton RJ, Pochan DP, Duncan RL, Jia X (2010) Effects of Matrix Composition, Microstructure, and Viscoelasticity on the Behaviors of Vocal Fold Fibroblasts Cultured in Three-Dimensional Hydrogel Networks. Tissue Eng A16:1247–1261
Kundu J, Poole-Warren LA, Martens P, Kundu SC (2012) Silk fibroin/poly(vinyl alcohol) photocrosslinked hydrogels for delivery of macromolecular drugs. Acta Biomater 8:1720–1729
Abbate V, Kong X, Bansal SS (2012) Photocrosslinked bovine serum albumin hydrogels with partial retention of esterase activity. Enzyme Microb Technol 50:130–136
Amruthwar SS, Janokar AV (2012) Preparation and characterization of elastin-like polypeptide scaffolds for local delivery of antibiotics and proteins. J Mater Sci: Mater Med 23:2903–2912
Elzoghby AO, Abo El-Fotoh WS, Elgindy NA (2011) Casein-based formulations as promising controlled release drug delivery systems. J Controlled Release 153:206–216
Xu J, Fan Z, Duan L, Gao G (2018) A tough, stretchable, and extensively sticky hydrogel driven by milk protein. Polymer Chemistry 9:2617–2624
Koh LD, Cheng Y, Teng CP, Khin YW, Loh XJ, Tee SY, Low M, Ye E, Yu HD, Zhang YW, Han MY (2015) Structures, mechanical properties and applications of silk fibroin materials. Prog Polym Sci 46:86–110
Crini G (2008) Kinetic and equilibrium studies on the removal of cationic dyes from aqueous solution by adsorption into a cyclodextrin polymer. Dyes Pigm 77:415–426
Ho Y, McKay G (1999) Pseudo-second order model for sorption processes. Process Biochem 34:451–465
Weber W, Morris J, Sanit J (1963) Kinetics of adsorption on carbon from solution. Journal Sanitary Engeering Division Proceedings, vol 89. American Society of Civil Engineers, pp 31–60
Tang H, Zhou W, Zhang L (2012) Adsorption isotherms and kinetics studies of malachite green on chitin hydrogels. J Hazard Mater 209:218–225
Guinesi LS, ETG C (2006) Influence of some reactional parameters on the substitution degree of biopolymeric Schiff bases prepared from chitosan and salicylaldehyde. Carbohydrate Polymers 65:557–561
Zhang R, Huang Z, Xue M, Yang J, Tan T (2011) Influence of some reactional parameters on the substitution degree of biopolymeric Schiff bases prepared from chitosan and salicylaldehyde. Carbohydr Polym 85:717–725
Hodge JE (1953) Chemistry of Browning Reaction in Model Systems. J Agric Food Chem 1:928–943
Bayramoglu G, Altintas B, Yakup AM (2009) Adsorption kinetics and thermodynamic parameters of cationic dyes from aqueous solutions by using a new strong cation-exchange resin. Chem Eng J 152:339
Guibal E (2004) Interactions of metal ions with chitosan-based sorbents: a review. Sep Purif Technol 38:43–74
Emik SM (2014) Preparation and characterization of an IPN type chelating resin containing amino and carboxyl groups for removal of Cu (II) from aqueous solutions. React Funct Polym 75:63–74
Bhattacharyya R, Ray SK (2014) Enhanced adsorption of synthetic dyes from aqueous solution by a semi-interpenetrating network hydrogel based on starch. J Ind Eng Chem 20:3714–3725
Al-Ghouti M, Khraisheh M, Ahmad SA (2005) Thermodynamic behavior and the effect of temperature on the removal of dyes from aqueous solution using modified diatomite: A kinetic study. J Colloid Interface Sci 287:6–13
Han X, Wang W, Ma X (2011) Adsorption characteristics of methylene blue into low cost biomass material lotus leaf. Chem Eng J 171:1–8
Anirudhan TS, Tharun AR (2012) Preparation and adsorption properties of a novel interpenetrating polymer network (IPN) containing carboxyl groups for basic dye from aqueous media. Chem Eng J 181:761–769
Acknowledgments
This work was supported by the Key project of Guangdong Natural Science Foundation of China (2017B030311007), the Special Funds for Public Welfare Research and Capacity Building of Guangdong Province in China (2016A020222017), and the Science and Technology Planning Project of Guangzhou, Guangdong Province, China (201607010249).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yi, J., Li, Y., Yang, L. et al. Kinetics and thermodynamics of adsorption of Cu2+ and methylene blue to casein hydrogels. J Polym Res 26, 235 (2019). https://doi.org/10.1007/s10965-019-1870-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-019-1870-x