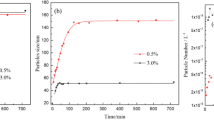
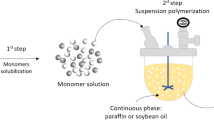
Abstract
Various heterogeneous polymerization techniques such as suspension, emulsion, dispersion, precipitation and seeded are employed for the synthesis of a wide variety of porous polymer particles. In present review, suspension polymerization technique is highlighted in detail with control of particle size, advantages and its applications. The aim of the review is to understand the basics of suspension polymerization for the synthesis of polystyrene cross-linked with divinylbenzene copolymer. Also, the effect of various synthesis parameters (agitation speed, temperature, initiator, cross-linker and diluent) on particle surface morphology, particle size and distribution, surface area and cross-linking density was reviewed and their application as catalyst support for various oxidation reactions.





Similar content being viewed by others
Abbreviations
- PS:
-
Polystyrene
- DVB:
-
Divinyl Benzene
- AIBN:
-
Azobiz isobutyl nitrile
- THF:
-
Tetrahydrofuran
- PMMA:
-
Polymethyl methacrylate
- PEG:
-
Polyethylene glycol
- BPO:
-
Benzoyl peroxide
- EGDMA:
-
Ethylene glycol monomethyl ether
- TEGDA:
-
Tetraethyleneglycol diacrylate
- PVC:
-
Polyvinylchloride
- PVA:
-
Polyvinyl acetate
- PAAm:
-
Polyacrylamide
- TFE:
-
Tetrafluoro-ethylene
- ACN:
-
acrylonitrile
- PE:
-
Polyethylene
- PAc:
-
Polyacrylates
References
Gokmen MT, Prez FED (2012) Porous polymer particles—A comprehensive guide to synthesis, characterization, functionalization and applications. Prog Polym Sci 37:365–405
Prasath RA, Gokmen MT, Espeel P, Prez FED (2010) Thiol-ene and thiol-yne chemistry in microfluidics: a straightforward method towards macroporous and nonporous functional polymer beads. Polym Chem 1:685
Qi S, Zhifeng D, Xiangju M, Shou XF (2015). Chem Soc Rev 44:6018
Dowding PJ, Goodwin JW, Vincent B (1998) The characterization of porous styrene–glycidyl methacrylate copolymer beads prepared by suspension polymerization. Colloids Surf A Physicochem Eng Asp 145:263–270
Heydarpoor S, Abbasi F, Jalili K, Najafpour M (2015) Synthesis of core-shell PS/PMMA expandable particles via seeded suspension polymerization. J Polym Res 22:151
El-Aassar MR, Soliman EA, Hashem AI, sun G, Amaly N (2017) Preparation and characterization of poly (styrene-co-Methacrylic acid) copolymer nanoparticles via precipitation polymerization. J Polym Res 24:207
Arshady R (1992) Suspension, emulsion, and dispersion polymerization: a methodological survey. Colloid Polym Sci 270:717–732
Tomovska R, Cal JC, Asua JM (2014). Wiley, New York, pp 59
Lima EV, Wood PE, Hamielec A (1997) An updated review on suspension polymerization. Ind Eng Chem Res 36:939–965
McNamara CA, Dixon MJ, Bradley M (2002) recoverable catalysts and reagents using recyclable polystyrene-based supports. Chem Rev 102:3275–3300
Hosseinzadeh S, Saadat Y, Abdolbaghi S, Taromi FA, Hosseinzadeh A (2014) Shape of the particles produced by seeded dispersion polymerization of styrene. Collod J 76:104–112
Kim JW, Suh K (2008) Monodisperse polymer particles synthesized by seeded polymerization techniques. J Ind Eng Chem 14:1–9
Kim JW, Suh K (2000) Monodisperse micron-sized polystyrene particles by seeded polymerization: effect of seed crosslinking on monomer swelling and particle morphology. Polymer 41:6181–6188
Lee K, Wi H (2010) Highly crosslinked micron-sized, monodispersed polystyrene particles by batch dispersion polymerization, Part 1: Batch, delayed addition, and seeded batch processes. J Appl Polym Sci 115:297–307
Qi Z, Han Y, Wang W, Song T, Chang J (2010). J Colloid Interface Sci 342:62
Kim JW, Ryu J, Suh K (2001) Monodisperse micron-sized macroporous poly(styrene- co -divinylbenzene) particles by seeded polymerization. Collod Polym Sci 279:146–152
Siddiqui MN, Redhwi HH, Vakalopoulou E, Tsagkalias I, Ioannidou MD, Achilias DS (2015) Synthesis, characterization and reaction kinetics of PMMA/silver nanocomposites prepared via in situ radical polymerization. Eur Polym J 72:256–269
Meouche W, Laatikainen K, Margaillan A, Silvonen T, Siren H, Sainio T, Beurroies I, Denoyel R, Branger C (2017) Effect of porogen solvent on the properties of nickel ion imprinted polymer materials prepared by inverse suspension polymerization. Eur Polym J 87:124–135
Ballard N, Aguirre M, Simula A, Leiza JR, Es S, Asua JM (2017) Nitroxide mediated suspension polymerization of methacrylic monomers. Chem Eng J 316:655–662
Asua JM (2018) Ostwald ripening of reactive costabilizers in miniemulsion polymerization. Eur Polym J 106:30–41
Schmidt BVKJ, Molinari V, Esposito D, Tauer K, Antonietti M (2017) Lignin-based polymeric surfactants for emulsion polymerization. Polymer 112:418–426
Sudjaipraparat N, Kaewsaneha C, Nuasaen S, Tangboriboonrat P (2017) One-pot synthesis of non-spherical hollow latex polymeric particles via seeded emulsion polymerization. Polymer 121:165–172
Silverstein MS (2017) Emulsion-templated polymers: Contemporary contemplations. Polymer 126:261–282
Cordoba CA, Collins SE, Passeggi Jr MCG, Vaillard SE, Gugliotta LM, Minari RJ (2018) Crosslinkable acrylic-melamine latex produced by miniemulsion polymerization. Prog Org Coat 118:82–90
Yamamoto T, Kawaguchi K, Takahashi Y (2016) Particle size control in the soap-free emulsion polymerization of styrene by an oil-soluble initiator with a weakly acidic water-soluble initiator. Colloids Surf A Physicochem Eng Asp 502:1–5
Oliveira PF, Machado RAF, Barth D, Acosta ED (2016) Dispersion polymerization of methyl methacrylate in supercritical carbon dioxide using vinyl terminated poly(dimethylsiloxane). Chem Eng Process 103:46–52
Chen R, Ren N, Jin X, Zhu X (2018) Stabilization capacity of PNIPAM microgels as particulate stabilizer in dispersion polymerization. Colloids Surf A Physicochem Eng Asp 538:789–794
Yang W, Hutchinson RA (2017) The influence of adding functionality to dispersant and particle core compositions in non-aqueous dispersion polymerization. React Funct Polym 114:31–37
Tan J, Li C, Dan S, Li H, Gu J, Zhang B, Zhang H, Zhang Q (2016). Chem Eng J 304:461
Wang X, Shen L, An Z (2018) Dispersion polymerization in environmentally benign solvents via reversible deactivation radical polymerization. Prog Polym Sci 83:1–27
Medeiros SF, Filizzola JOC, Oliveira PFM, Silva TM, Lara BR, Lopes MV, Bergmann BR, Elaissari A, Santos AM (2016) Fabrication of biocompatible and stimuli-responsive hybrid microgels with magnetic properties via aqueous precipitation polymerization. Mater Lett 175:296–299
Zhang H (2013) Controlled/“living” radical precipitation polymerization: a versatile polymerization technique for advanced functional polymers. Eur Polym J 49:579–600
Wang X, Huang P, Ma X, Du X, Lu X (2018) Magnetic mesoporous molecularly imprinted polymers based on surface precipitation polymerization for selective enrichment of triclosan and triclocarban. J Chromatogr A 1537:35–42
Huang H, Wang H, Wu Y, Shi Y, Deng J (2018) Chiral, crosslinked, and micron-sized spheres of substituted polyacetylene prepared by precipitation polymerization. Polymer 139:76–85
Jolly HC, Guillot P, Mignard E (2018) Supercritical continuous precipitation polymerization of acrylic acid in a droplet-based millifluidic device. Chem Eng J 334:389–399
Chaitidou S, Kotrotsiou O, Kotti K, Kammona O, Bukhari M, Kiparissides C (2008) Precipitation polymerization for the synthesis of nanostructured particles. Mater Sci Eng B 152:55–59
Kao YC, Whang WT, Chen YC, Chen KC (2017) Effect of crosslinking agents on the dispersive behaviour of polymer particles in seed swelling polymerisation. J Ind Eng Chem 51:216–222
Pei X, Zhai K, Liang X, Deng Y, Xu K, Tan Y, Yao X, Wang P (2018) Fabrication of shape-tunable macroparticles by seeded polymerization of styrene using non-cross-linked starch-based seed. J Colloid Interface Sci 512:600–608
Zhang Z, Shao H, Zhou X, Zhao L, Liu H, Ji X, Liu H (2017) Controllable synthesis of anisotropic silica/polymer composite particles via seeded dispersion polymerization. Mater Chem Phys 195:105–113
Yao Y, Cao Z, Shang Y, Chen Q, Yang L, Zhang Y, Qi D (2017) Preparation of polymeric/inorganic nanocomposite particles in miniemulsions: II. Narrowly size-distributed polymer/SiO2 nanocomposite particles. Colloids Surf A Physicochem Eng Asp 530:104–116
Kim D, Lee DY, Lee K, Choe S (2009) Effect of crosslinking agents on the morphology of polymer particles produced by one-step seeded polymerization. Macromol Res 17:250–258
Park J, Kim Y, Yoon H, Jun LBY (2011) Preparation of pore size controllable macroporous polymer beads. J Ind Eng Chem 17:794–798
Wang H, Xie G, Fang M, Ying Z, Tong Y, Zeng Y (2017) Mechanical reinforcement of graphene/poly(vinyl chloride) composites prepared by combining the in-situ suspension polymerization and melt-mixing methods. Composites Part B 113:278–284
Kiatkamjornwong S, Chientachakul P, Prasassarakich P, Damronglerd S (2001) Kinetic studies on styrene-divinylbenzene copolymerization by suspension technique. J Appl Polym Sci 82:1521–1540
Ober CK, Hair ML (1987). J Appl Polym Sci 25:1395
Kichatov BV, Korshunov AM, Assorova PV (2003). Theor Found Chem Eng 37:305
Liu Q, Wang L, Xiao A, Yu H (2010) The spherical cleavage behavior of Polydivinylbenzene during suspension polymerization. Des Mon Polym 13:369–375
Nunes DSS, Coutinho FMB (2002) Acrylonitrile–divinylbenzene copolymer beads: influence of pre-polymerization step, stirring conditions and polymerization initiator type on the polymer particle characteristics. Eur Polym J 38:1159–1165
Rodrigo R, Toro CA, Cuellar J (2013) Morphological characteristics of poly(styrene-co-divinylbenzene) microparticles synthesized by suspension polymerization. Powder Technol 247:279–288
Okudaira G, Kamogawa K, Sakai T, Sakai H, Abe M (2003). J Oleo Sci 53:167
Svec F, Frechet JMJ (1995) Temperature, a Simple and Efficient Tool for the Control of Pore Size Distribution in Macroporous Polymers. Macromol 28:7580–7582
Hulubei C, Vlad CD, Stoica I, Popovici D, Lisa G, Nica SL, Barzic AL (2014) New polyimide-based porous crosslinked beads by suspension polymerization: physical and chemical factors affecting their morphology. J Polym Res 21:514
Hoffman F, Delbruch K (1909) Patent (Ger.) No. 250 690, Farbenfabriken Bayer, Germany
Svec F, Frechet JMJ (1995). Biotechnol Bioeng 48:476
Viklund C, Svec F, Frechet JMJ, Irgum K (1996) Monolithic, “molded”, porous materials with high flow characteristics for separations, catalysis, or solid-phase chemistry: control of porous properties during polymerization. Chem Mater 8:744–750
Petro M, Svec F, Frechet JMJ (1996) Immobilization of trypsin onto “molded” macroporous poly(glycidyl methacrylate-co-ethylene dimethacrylate) rods and use of the conjugates as bioreactors and for affinity chromatography. Biotechnol Bioeng 49:355–363
Petro M, Svec F, Frechet JMJ (1996) Molded continuous poly(styrene-co-divinylbenzene) rod as a separation medium for the very fast separation of polymers comparison of the chromatographic properties of the monolithic rod with columns packed with porous and non-porous beads in high-performance liquid chromatography of polystyrenes. J Chromatogr A 752:59–66
Xie SF, Svec F, Frechet JMJ (1997) Preparation of porous hydrophilic monoliths: Effect of the polymerization conditions on the porous properties of poly (acrylamide-co-N,N?-methylenebisacrylamide) monolithic rods. J Polym Sci Part A Polym Chem 35:1013–1021
Peters EC, Svec F, Frechet JMJ (1997) Preparation of Large-Diameter “Molded” Porous Polymer Monoliths and the Control of Pore Structure Homogeneity. Chem Mater 9:1898–1902
Xie SF, Svec F, Frechet JMJ (1997) Rigid porous polyacrylamide-based monolithic columns containing butyl methacrylate as a separation medium for the rapid hydrophobic interaction chromatography of proteins. J Chromatogr A 775:65–72
Peters EC, Petro M, Svec F, Frechet JMJ (1998) Molded rigid polymer monoliths as separation media for capillary electrochromatography. 1. fine control of porous properties and surface chemistry. Anal Chem 70:2288–2295
Alexopoulos AH, Kiparissides C (2007) On the prediction of internal particle morphology in suspension polymerization of vinyl chloride. Part I. Chem Eng Sci 62:3970–3983
Tan L, Tan B (2016) Hypercrosslinked porous polymer materials: design, synthesis, and applications. Chem Soc Rev 46:3322–3356. https://doi.org/10.1039/c6cs00851h
Bauer W, Lauth H (1931) Patent (Ger.) No. 656, 134, Rohm and Hass, Darmstadt
Hosenstein WP, Mark H (1946) Polymerization of olefins and diolefins in suspension and emulsion. Part I. Int J Polym Sci 1:127–145
Trommsdorff E, Kohle H, Lagally P (1948) Zur polymerisation des methacrylsäuremethylesters1. Macromol Chem 1:169–198
Munzer M, Trommsdorff E (1997). High Polym 29:106
Yuan HG, Kalfas G, Ray WH (1991) Suspension polymerization. J Macromol Sci Rev Macrolol Chem Phys C 31:215–299
Okay O (2000) Macroporous copolymer networks. Prog Polym Sci 25:711–779
Kita R, Svec F, Frechet JMJ (2001) Hydrophilic polymer supports for solid-phase synthesis: preparation of Poly(ethylene glycol) methacrylate polymer beads using “classical” suspension polymerization in aqueous medium and their application in the solid-phase synthesis of hydantoins. J Comb Chem 3:564–571
Slater M, Snauko M, Svec F, Frechet JMJ (2006) “Click chemistry” in the preparation of porous polymer-based particulate stationary phases for μ-HPLC separation of peptides and proteins. Anal Chem 78:4969–4975
Mohammed ML, Mbeleck R, Saha B (2015) Efficient and selective molybdenum based heterogeneous catalyst for alkene epoxidation using batch and continuous reactors. Polym Chem 6:7308–7319
Mouradzadegun A, Mostafavi MA (2016) Copper-loaded hypercrosslinked polymer decorated with pendant amine groups: a green and retrievable catalytic system for quick [3 + 2] Huisgen cycloaddition in water. RSC Adv 6:42522–42531
Saadati F, Khani N, Rahmani M, Piri F (2016) Preparation and characterization of nanosized copper (II) oxide embedded in hyper-cross-linked polystyrene: highly efficient catalyst for aqueous-phase oxidation of aldehydes to carboxylic acids. Catal Commun 79:26–30
Taguchi Y, Suzuki T, Saito N, Yokoyama H, Tanaka M (2017) Preparation of polymer composite particles by phase separation followed by suspension polymerization. Open J Compos Mater 7:1–13
Alva G, Lin Y, Liu L, Fang G (2017) Synthesis, characterization and applications of microencapsulated phase change materials in thermal energy storage: A review. Energy Buil 144:276–294
Dowding PJ, Vincent B (2000) Suspension polymerisation to form polymer beads. Colloids Surf A Physicochem Eng Asp 161:259–269
Liang YC, Svec F, Frechet JMJ (1997) Preparation and functionalization of reactive monodisperse macroporous poly(chloromethylstyrene-co-styrene-co-divinylbenzene) beads by a staged templated suspension polymerization. J Polym Sci Part A Polym Chem 35:2631–2643
Arshady R, Ledwith A (1983). React Polym 1:159
Minami H, Kojima A, Suzuki T (2017) Preparation of flattened cross-linked hollow particles by suspension polymerization in a solid dispersion medium. Langmuir 33:1541–1546
Erbay E, Bilgic T, Karali M, Savasci OT (1992) Polystyrene suspension polymerization: the effect of polymerization parameters on particle size and distribution. Polym-Plast Technol Eng 31:589–605
Lima EV, Hamielec AE, Wood PE (1994) Auto-acceleration effect in free radical polymerization. A comparison of the CCS and MH models. Polym React Eng 2:17–85
Villalobos MA, Hamielec AE, Wood PE (1993) Bulk and suspension polymerization of styrene in the presence of n-pentane. An evaluation of monofunctional and bifunctional initiation. J Appl Polym Sci 50:327–343
Grochowicz M, Gawdzik B (2013) Preparation and characterization of porous crosslinked microspheres of new aromatic methacrylates. J Porous Mater 20:339–349
Sharma S, Sinha S, Biswas P, Maurya MR, Chand S (2013) Oxidation of styrene over polymer- and nonpolymer-anchored Cu(II) and Mn(II) complex catalysts. J Appl Polym Sci 127:3424–3434
Sharma S, Sinha S, Chand S (2012) Polymer anchored catalysts for oxidation of styrene using TBHP and molecular oxygen. Ind Eng Chem Res 51:8806–8814
Sarkar S, Guibal E, Quignard F, SenGupta AK (2012) Polymer-supported metals and metal oxide nanoparticles: synthesis, characterization, and applications. J Nanopart Res 14:715
Dioos BML, Vankelecom IFJ, Jacobs PA (2006) Aspects of Immobilisation of catalysts on polymeric supports. Adv Synth Catal 348:1413–1446
Tank R, Gupta DC (2009) Modification of styrene-divinyl benzene copolymers using monoacrylates as ter-monomer. J Porous Mater 16:387–392
Apostolidou C, Stamatoudis M (1990) On particle size distribution in suspension polymerization of styrene. Collect Czechoslov Chem Commun 55:2244–2251
Portnikov D, Kalman H (2018) The effect of temperature on the mechanical characteristics of individual particles. Powder Technol 336:393–405
Azouz KB, Bekkour K, Dupuis D (2016) Influence of the temperature on the rheological properties of bentonite suspensions in aqueous polymer solutions. Appl Clay Sci 123:92–98
Ng WS, Cooper L, Connal LA, Forbes E, Jameson GJ, Franks GV (2018) Tuneable collector/depressant behaviour of xanthate-functional temperature-responsive polymers in the flotation of copper sulfide: effect of shear and temperature. Miner Eng 117:91–99
Zhenga T, Pilla S (2018) Encapsulation of hydrophilic payload by PU-PMF capsule: effect of melamine-formaldehyde pre-polymer content, pH and temperature on capsule morphology. Colloids Surf A Physicochem Eng Asp 542:59–67
Gritskova IA, Zhachenkov SV, Tsarkova MS, Levachev SM, Simakova GA, Khaddazh M, Prokopov NI (2011). Polymer Sci 53:568
Mane S, Ponrathnam S, Chavan N (2015). Can Chem Trans 3:473
Mane S, Ponrathnam S, Chavan N (2016). Can Chem Trans 4:192
Luz CTL, Coutinho FMB (2001). Polymer 42:4931
Daminova SS, Kadirova ZC, Sharipov KT, Stoyko OV, Chepulsky SA, Adewuyi A, Hojamberdiev M (2017) Diisopropyldithiophosphoric acid-impregnated macroporous non-ionogenic styrene-divinylbenzene polymeric sorbent (Porolas) for effective copper extraction. J Ind Eng Chem 55:204–214
Lu L, Jiang C, Xiufang W, Pihui P, Zhuoru Y (2006). Chin J Chem Eng 14:471
Kiatkamjornwong S, Traissaranapong S, Prasassarakich P (1999). J Porous Mater 6:215–229
Aungsurpravate O, Kangwansupamonkon W, Chavasiri W, Kiatkamjornwong S (2007). Polym Eng Sci 447
Mane S (2016). Can Chem Trans 4:210
Hao D, Gong F, Wei W, Hu G, Ma G, Su Z (2008) Porogen effects in synthesis of uniform micrometer-sized poly(divinylbenzene) microspheres with high surface areas. J Colloid Interface Sci 323:52–59
Calabrase RV, Chang TPK, Dang PT (1986) Drop breakup in turbulent stirred-tank contactors. Part I: Effect of dispersed-phase viscosity. AICHE J 32:657–666
Borwankar RP, Chung SI, Wasan DT (1986). J Appl Polym Sci 329:5749
Bourne JR, Baldyga (1994). J Chem Eng Sci 499:1077
Coulaloglou CA, Tavlarides LL (1976). AICHE J 229:289
Doulah MS (1975). Ind Eng Chem Fundam 149:137
Chaudhary V, Sweta (2016). IJSER 7:1743–1748
Chaudhary V, Sweta (2017). J Porous Mater 24:741–749
Fu Y, Xu L, Shen H, Yang H, Zhang F, Zhu W, Fan M (2016) Tunable catalytic properties of multi-metal–organic frameworks for aerobic styrene oxidation. Chem Eng J 299:135–141
Tamami B, Ghasemi S (2011) Modified crosslinked polyacrylamide anchored Schiff base–cobalt complex: a novel nano-sized heterogeneous catalyst for selective oxidation of olefins and alkyl halides with hydrogen peroxide in aqueous media. Appl Catal A Gen 393:242–250
Godhani DR, Nakum HD, Parmar DK, Mehta JP, Desai NC (2016) Tuning of the reaction parameters to optimize allylic oxidation of cyclohexene catalyzed by zeolite-Y entrapped transition metal complexes. J Mol Catal A Chem 415:37–55
Sherrington DC (1980). British Polym J 70
Kralik M, Corain B, Zecca M (2000). Chem Pap 54:254
Arnold U (2008) Mechanisms in homogeneous and heterogeneous epoxidation catalysis, p 387
Collma JP, Kosydar KM, Bressan M, Lamanna W, Garrett T (1984) Polymer-bound substrates: a method to distinguish between homogeneous and heterogeneous catalysis. J Am Chem Soc 106:2569–2579
Gravert DJ, Janda KD (1997) Organic synthesis on soluble polymer supports: liquid-phase methodologies. Chem Rev 97:489–510
Merrifield B (1984) The role of the support in solid phase peptide synthesis. British Polym J 16:173–178
Yamaguchi K, Mizuno N (2003) Scope, kinetics, and mechanistic aspects of aerobic oxidations catalyzed by ruthenium supported on alumina. Chem Eur J 9:4353–4361
Abad A, Concepcion P, Corma A, Garcia H (2005) A collaborative effect between gold and a support induces the selective oxidation of alcohols. Angew Chem Int Ed 44:4066–4069
Jamwal N, Gupta M, Paul S (2008) Hydroxyapatite-supported palladium (0) as a highly efficient catalyst for the Suzuki coupling and aerobic oxidation of benzyl alcohols in water. Green Chem 10:999
Renuka MK, Gayathri V (2018) A polymer supported Cu(II) catalyst for oxidative amidation of benzyl alcohol and substituted amines in TBHP/H 2 O. Catal Commun 104:71–77
Kaboudin B, Khanmohammadi H, Kazemi F (2017) Polymer supported gold nanoparticles: synthesis and characterization of functionalized polystyrene-supported gold nanoparticles and their application in catalytic oxidation of alcohols in water. Appl Surf Sci 425:400–406
Wang Y, Huang J, Xia X, Peng X (2018) Fe–Co/sulfonated polystyrene as an efficient and selective catalyst in heterogeneous Baeyer–Villiger oxidation reaction of cyclic ketones. J Saudi Chem Soc 22:129–135
Gupta KC, Sutar AK (2008) Polymer supported catalysts for oxidation of phenol and cyclohexene using hydrogen peroxide as oxidant. J Mol Catal A Chem 280:173–185
Hatefi M, Moghadam M, Sheikhshoaei I, Mirkhani V, Tangestaninejad S, Baltork IM, Kargar H (2009) Ru(salophen)Cl supported on polystyrene-bound imidazole: an efficient and robust heterogeneous catalyst for epoxidation of alkenes with sodium periodate. Appl Catal A 370:66–71
Krishnan GR, Sreekumar K (2009) Polystyrene-supported poly(amidoamine) dendrimer–manganese complex: synthesis, characterization and catalysis. Appl Catal A 353:80–86
Chang Y, Lv Y, Lu F, Zha F, Lei Z (2010) Efficient allylic oxidation of cyclohexene with oxygen catalyzed by chloromethylated polystyrene supported tridentate Schiff-base complexes. J Mol Catal A Chem 320:56–61
Tangestaninejad S, Moghadam M, Mirkhani V, Baltork IM, Torki M (2011) Preparation and characterization of molybdenum hexacarbonyl encapsulated in polystyrene and its application as an efficient and reusable catalyst for epoxidation of alkenes with tert-BuOOH. C R Chimie 14:604–610
Mohammed ML, Mbeleck R, Patel D, Niyogi D, Sherrington DC, Saha B (2015) Greener and efficient epoxidation of 4-vinyl-1-cyclohexene with polystyrene 2-(aminomethyl)pyridine supported Mo(VI) catalyst in batch and continuous reactors. Chem Eng Res Des 94:194–203
Zhu Y, Zhao W, Hosmane NS (2015) Direct synthesis of carboranylpolystyrene and their applications for oxidation resistance of graphene oxides and catalyst support. J Organomet Chem 798:80–85
Sharma AS, Kaur H (2017) Au NPs@ polystyrene resin for mild and selective aerobic oxidation of 1,4 dioxane to 1,4 dioxan-2-ol. Catal Commun 90:56–59
Khatun R, Biswas S, Ghosh S, Islam SM (2018) Polymer-anchored [Fe(III)Azo] complex: an efficient reusable catalyst for oxidative bromination and multi-components reaction for the synthesis of spiropiperidine derivatives. J Organomet Chem 858:37–46
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chaudhary, V., Sharma, S. Suspension polymerization technique: parameters affecting polymer properties and application in oxidation reactions. J Polym Res 26, 102 (2019). https://doi.org/10.1007/s10965-019-1767-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-019-1767-8