Abstract
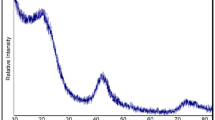
Phenolic resins are important materials in various application sectors due to their outstanding mechanical and thermal properties. However, phenolic resins are known as typical non-graphitized carbon precursor. In this work, important parameters related to the synthesis of PRs were studied: the concentration of the catalyst and the molar ratio between phenol and formaldehyde. The structural information was performed by Fourier Transformation Infrared Spectroscopy and solid state nuclear magnetic resonance of carbon analysis to define mainly between novolac or resol resin. The thermal characteristics of the resins were studied for TGA and their tendencies for graphitization were verified by powder x-ray diffraction and Raman spectroscopy and SEM/HRTEM analysis. As a conclusion, it was verified that the phenolic resins prepared with excess of formaldehyde in acidic medium can present different properties according to their composition, which allows for qualifying them as potential materials for different applications in the technological area that involves graphitized carbon.












Similar content being viewed by others
References
Hirano K, Asami M (2013) Phenolic resins-100 years of progress and their future. React Funct Polym 73:256–269
Pulci G, Tirillò J, Marra F, Fossati F, Bartuli C, Valente T (2010) Carbon-phenolic ablative materials for re-entry space vehicles: manufacturing and properties. Compos Part A 41:1483–1490
Winya N, Boonpan A, Prapunkarn K (2013) Study of factors affecting the ablation rate of phenolic resin / Fiber glass. Int J Chem Eng Appl 4:234–237
Soutis C (2005) Carbon fiber reinforced plastics in aircraft construction. Mater Sci Eng A 412:171–176
Pilato L (2010) Phenolic resins: a century of progress. Springer-Velag Berlin Heidelberg, New Jersey
Binner J, Hogg PJ, Sweeney J (1993) Advanced materials sourcebook. Elvesier Advanced Technology, Oxford
Lei S, Guo Q, Zhang D, Shi J, Liu L, Wei X (2010) Preparation and properties of the phenolic foams with controllable nanometer pore structure. J Appl Polym Sci 117:3345–3550
Shen H, Lavoie AJ, Nutt SR (2003) Enhanced peel resistance of fiber reinforced phenolic foams. Compos Part A 34:941–948
Wang Y, Chen L, Xu T, Yan Y, Gu J, Yun J, Feng J (2017) High char yield novolac modified by Si-B-N-C precursor: thermal stability and structural evolution. Polym Degrad Stab 137:184–196
Bitencourt CS, Pandolfelli VC (2013) Resinas termofixas e a produção de refratários contendo carbono: base teórica e insights para futuros desenvolvimentos. Cerâmica 59:1–26
Mano EB (1991) Polímeros como Materiais de Engenharia, São Paulo
(1999) Suppliers team up for offshore breakthrough. Reinforced plastics. 43(7):16. https://doi.org/10.1016/S0034-3617(99)80058-7
(1996) Modified phenolic pipe heats up the offshore market. Reinf Plast 40:50–51. https://doi.org/10.1016/S0034-3617(98)80227-0
Zhang J, Mei G, Xie Z, Zhao S (2016) Curing mechanism of phenolic resin binder for oxide-carbon refractories. ISIJ Int 56:44–49
Gardziella A, Pilato LA, Knop A (2000) Phenolic resins: chemistry, applications, standardization, safety and ecology2nd edn. Springer-Verlag, Berlin Heidelberg
Chen H, Zhou M, Wang Z, Zhao S, Guan S (2014) Rich nitrogen-doped ordered mesoporous phenolic resin-based carbon for supercapacitors. Electrochim Acta 148:187–194
Wang M, Fan L (2013) Silicon/carbon nanocomposite pyrolyzed from phenolic resin as anode materials for lithium-ion batteries. J Power Sources 244:570–574
Haddadi SA, Mahdavian-Ahadi M, Abbasi F (2014) Effect of Nanosilica and boron carbide on adhesion strength of high temperature adhesive based on phenolic resin for graphite bonding. Ind Eng Chem Res 53:11747–11754
Huang G, Liu H, Yang L, He Y, Xia X, Chen H (2016) Pyrolysis behavior of graphene/phenolic resin composites. Carbon NY 98:733–736
Lin C, Lee H, Chen J (2013) Synthesis and characterization of molybdenum/phenolic resin composites binding with aluminum nitride particles for diamond cutters. Appl Surf Sci 284:297–307
Pérez JM, Oliet M, Alonso MV, Rodríguez F (2009) Cure kinetics of lignin-novolac resins studied by isoconversional methods. Thermochim Acta 487:39–42
Zhou J, Yao Z, Chen Y, Wei D, Wu Y, Xu T (2013) Mechanical and thermal properties of graphene oxide/phenolic resin composite. Polym Compos 34:1245–1249
Kapadia M, Patel M, Patel G, Joshi J (2008) La (III) polychelates of phenolic resin: synthesis, characterization and ion-exchange study. J Polym Res 15:285–293
Patel M, Kapadia M, Joshi J (2009) Polymer-metal complexes of phenolic resin with ln (III): thermal, catalytic and antimicrobial aspects. J Polym Res 16:755–765
Feng J, Chen L, Gu J, He Z, Yun J, Wang X (2016) Synthesis and characterization of aryl boron-containing thermoplastic phenolic resin with high thermal decomposition temperature and char yield. J Polym Res 23(97):1–7
Desai TG, Lawson JW, Keblinski P (2011) Modeling initial stage of phenolic pyrolysis: graphitic precursor formation and interfacial effects. Polymer (Guildf) 52:577–585
Chu L, Wang J (2011) Nitrogen removal using biodegradable polymers as carbon source and biofilm carriers in a moving bed biofilm reactor. Chem Eng J 170:220–225
Takami T, Seino R, Yamazaki K, Ogino T (2014) Graphene film formation on insulating substrates using polymer films as carbon source. J Phys D Appl Phys 47:94015
Fitzer E, Schaefer W, Yamada S (1969) The formation of glasslike carbon by pyrolysis of polyfurfuryl alcohol and phenolic resin. Carbon NY 7:643–648
Rand B, McEnaney B (1985) Carbon binders from polymeric resins and pitch, part I - pyrolysis behaviour and structure of the carbons. Br Ceram Trans J 84:157–165
Borges SG (2004) Síntese e Caracterização de Resinas Fenólicas Líquidas do Tipo Novolaca Aplicáveis no Processo de Pultrusão Síntese e Caracterização de Resinas Fenólicas Líquidas do Tipo Novolaca Aplicáveis no Processo de Pultrusão. Universidade Federal do Rio Grande do Sul, UFRGS, Porto Alegre
Grenier-Loustalot MF, Larroque S, Grenier P, Leca JP, Bedel D (1994) Phenolic resins: 1. Mechanisms and kinetics of phenol and of the first polycondensates towards formaldehyde in solution. Polymer (Guildf) 35:3046–3054
Grenier-Loustalot MF, Larroque S, Grande D, Grenier P, Bedel D (1996) Phenolic resins: 2. Influence of catalyst type on reaction mechanisms and kinetics. Polymer (Guildf) 37:1363–1369
Grenier-Loustalot MF, Larroque S, Grenier P, Bedel D (1996) Phenolic resins: 3. Study of the reactivity of the initial monomers towards formaldehyde at constant pH, temperature and catalyst type. Polymer (Guildf) 37:939–953
Grenier-Loustalot MF, Larroque S, Grenier P, Bedel D (1996) Phenolic resins: 4. Self-condensation of methylolphenols in formaldehyde-free media. Polymer (Guildf) 37:955–964
Sojka SA, Wolfe RA, Guenther GD (1981) Formation of phenolic resins: mechanism and time dependence of the reaction of phenol and hexamethylenetetramine as studied by carbon-13 nuclear magnetic resonance and Fourier transform infrared spectroscopy. Macromolecules 14:1539–1543
Crompton TR (2012) Thermal stability of polymers, Smithers Rapra technology ltd, Shawbyry, Shewsbury, Shrosphire. SY4 4NR, UK
Lee Y, Kim D, Kim H, Hwang T, Rafailovich M, Sokolov J (2003) Activation energy and curing behavior of Resol- and Novolac-type phenolic resins by differential scanning calorimetry and thermogravimetric analysis. J Appl Polym Sci 89:2589–2596
Markovic S, Dunjic B, Zlatanic A, Djonlagic J (2001) Dynamic mechanical analysis study of the curing of phenol-formaldehyde novolac resins. J Appl Polym Sci 81:1902–1913
Carotenuto G, Nicolais L (1999) Kinetic study of phenolic resin cure by IR spectroscopy. J Appl Polym Sci 74:2703–2715
Wan J, Wang S, Li C, Zhou D, Chen J, Liu Z, Yu L, Fan H, Li BG (2012) Effect of molecular weight and molecular weight distribution on cure reaction of novolac with hexamethylenetetramine and properties of related composites. Thermochim Acta 530:32–41
Kumar A, Gupta SK, Kumar B, Sumo N (1983) Molecular weight distributions in novolac type phenol-formaldehyde polymerizations. Polymer (Guildf) 24:1180–1187
Silverstein R, Webster FX, Kiemle DJ (2006) Identificação Espectrométrica de Compostos Orgânicos7.ed edn. Editora LTC, Rio de Janeiro
Adabbo HE, Williams RJJ (1982) Curing of Novolacs with paraformaldehyde. J Appl Polym Sci 27:893–901
Hatfield GR, Maciel GE (1987) Solid-state NMR study of the hexamethylenetetramine curing of phenolic resins. Macromolecules 20:608–615
Lenghaus K, Qiao GGH, Solomon DH (2001) 3,5-Dimethylphenol resole resins: their structure and mechanism of thermal decomposition leading to graphitisation. Polymer (Guildf) 42:7523–7529
Wang Y, Wang S, Bian C, Zhong Y, Jing X (2015) Effect of chemical structure and cross-link density on the heat resistance of phenolic resin. Polym Degrad Stab 111:239–246
Jones R, Jenkins GM (1976) 9.Volume changes in phenolic resin during carbonization. Carbon N Y 76:27.6–27.7
Ayadi S, Jedidi I, Rivallin M, Gillot F, Lacour S, Cerneaux S, Cretin M, Amar RB (2013) Elaboration and characterization of new conductive porous graphite membranes for electrochemical advanced oxidation processes. J Membr Sci 446:42–49
Mukhopadhyay P, Gupta RK (2013) 1.4 structural and mechanical characterization of graphite, in: graph. Graphene their Polym. Nanocomposites. CRC PRESS - Taylor & Francis Group, Boca Raton, p 21
Oya A, Yamashita R, Otani S (1979) Catalytic graphitization of carbons by borons. Fuel 58:495–500
Liu C, Li K, Li H, Zhang S, Zhang Y (2014) The effect of zirconium incorporation on the thermal stability and carbonized product of phenol-formaldehyde resin. Polym Degrad Stab 102:180–185
Cuesta A, Dhamelincourt P, Laureyns J, Martínez-Alonso A, Tascón JMD (1994) Raman microprobe studies on carbon materials. Carbon NY 32:1523–1532
Cuesta A, Dhamelincourt P, Laureyns J, Martínez-Alonso A, Tascón JMD (1998) Comparative performance of X-ray diffraction and Raman microprobe techniques for the study of carbon materials. J Mater Chem 8:2875–2879
Acknowledgements
The authors would like to thank Embrapa Instrumentação for SEM and NMR measurements, Laboratório de Cristalografia (UNIFAL-MG) for the XRD measurements, Laboratório de Materiais Fotônicos (LAMF/ UNESP) for the Raman Measurements and Laboratório de Caracterização Estrutural (LCE/UFSCar).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Renda, C.G., Bertholdo, R. Study of phenolic resin and their tendency for carbon graphitization. J Polym Res 25, 241 (2018). https://doi.org/10.1007/s10965-018-1635-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-018-1635-y