Abstract
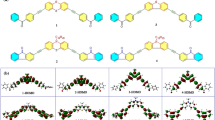
A series of copolymers Poly (TBA-CA-St-ASM)(PTCSA) were synthesized via precipitation polymerization by using tert-Butyl acrylate (TBA), Styrene (St), p-acetoxy styrene (ASM), and cedryl methacrylate (CA) as co-monomer. Then, Poly (TBA-CA-St-HS)(PTCSH) was prepared in the presence of sodium methoxide in methanol. The fourier transfer infrared (FT-IR) spectra and proton nuclear magnetic resonance (1H–NMR) spectra indicated that the synthesis was successful. The molecular weight, glass transition temperature (Tg) and thermal decomposition temperature (T(10%)) of the copolymers increased with the addition of CA. Moreover, a positive-tone chemically amplified Krypton Fluoride (KrF) photoresist was prepared, and the photolithography performance of the photoresist was evaluated using a KrF laser exposure system, the result showed that the resolution could reach the level of 0.25 μm.








Similar content being viewed by others
References
Chang S, Yang JH, Chien JH, et al. (2013) Synthesis of a novel alkaline-developable photosensitive copolymer based on MMA,MAA,SM,and 2-HEMA-grafted GMA copolymer for an innovative photo-imageable dry-peelable temporary protective plastisol. J Polym Res 20(4):1–11
Singh V, Satyanarayana VSV, Sharma SK, et al. (2014) Towards novel non-chemically amplified (n-CARS) negative resists for electron beam lithography applications. J Mater Chem C 2(12):2118–2122
Li H, Liu J, Zheng X, et al. (2016) Synthesis of chemically amplified photoresist polymer containing four (meth) acrylate monomers via RAFT polymerization and its application for KrF lithography. J Polym Res 23(5):1–7
Lee CK, Hwang FH, Chen CC, et al. (2012) Preparation and characterization of nanosilica-filled color resist. Adv Polym Technol 31(2):163–171
Accoto C, Qualtieri A, Pisanello F, et al. (2015) Two-photon polymerization lithography and laser Doppler Vibrometry of a SU-8-based suspended microchannel resonator. J Microelectromech Syst 24(4):1038–1042
Ito H (2005) Chemical amplification resists for microlithography. Adv Polym Sci 172:44
Ito H (2003) Chemical amplification resists: inception, implementation in device manufacture, and new developments. J Polym Sci A Polym Chem 41(24):3863–3870
Przybilla KJ, Roeschert H, Spiess W, et al. (1991) Progress in DUV resins. Advances in Resist Technology and Processing VIII 1466:174–187
Wei Q, Hu F, Wang L (2015) Formation of Nanotunnels inside a resist film in laser interference lithography. Langmuir 31(19):5464–5468
Liu J, Qiao Y, Liu Z, Wang L (2014) The preparation of a novel polymeric sulfonium salt photoacid generator and its application for advanced photoresists. RSC Adv 4(40):21093–21100
Frechet JM, Eichler E, Ito H, et al. (1983) Poly (p-tert-butoxycarbonyloxystyrene): a convenient precursor to p-hydroxystyrene resins. Polymer 24(8):995–1000
Ahn KD, Koo DI, Kim SJ (1991) T-BOC maleimide copolymers for thermally stable deep UV resists by chemical amplification. J Photopolym Sci Technol 4(3):433–443
Varanasi PR, Cornett KM, Katnani AD (1998) Acid-sensitive arylether-protected poly (4-hydroxystyrene) derivatives for chemically amplified deep-UV positive resists. Advances in Resist Technology and Processing XV 3333:512–523
Ota T, Ikezaki Y, Kajita T, et al. (1994) Effects of deprotected species on chemically amplified resist systems. Advances in Resist Technology and Processing XI 2195:74–83
Funhoff DJ, Binder H, Schwalm R (1992) Deep-UV resists with improved delay capabilities. Advances in Resist Technology and Processing IX 1672:46–55
Thackeray JW, Adams T, Fedynyshyn TH, et al. (1993) Deep UV photoresists: Photospeed, resolution, and environmental stability tradeoffs. J Photopolym Sci Technol 6(4):645–656
Ito H, Breyta G, HOFER D, et al. (1994) Environmentally stable chemical amplification positive resist: principle, chemistry, contamination resistance, and lithographic feasibility. J Photopolym Sci Technol 7(3):433–447
Tanabe T, Kobayashi Y, Tsuji A (1996) PED-stabilized chemically amplified photoresist. Advances in Resist Technology and Processing XIII 2724:61–69
Ito H, Alexander DF, Breyta G (1997) Dissolution kinetics and PAG interaction of phenolic resins in chemically amplified resists. J Photopolym Sci Technol 10(3):397–407
Zhang C, Zhou Y, Liu Q, et al. (2011) Facile synthesis of hyperbranched and star-shaped polymers by RAFT polymerization based on a polymerizable trithiocarbonate. Macromolecules 44(7):2034–2049
Gokan H, Esho S, Ohnishi Y (1983) Dry etch resistance of organic materials. J Electrochem Soc 130(1):143–146
Acknowledgments
This work was supported by Innovation Foundation of Jiangsu (No.BY2015019-14), the National Science and Technology Major Project of China (No.2010ZX02304), the National Nature Science Foundation of Jiangsu Province (No.BK20140160), the Fundamental Research Funds for the Central Universities (No. JUSRP51719A) and Suzhou Rui Hong Electronic Chemicals Co., Ltd.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Zheng, X., Ji, C., Zeng, Q. et al. Synthesis of novel copolymer based on precipitation polymerization and its application in positive-tone photoresist. J Polym Res 24, 198 (2017). https://doi.org/10.1007/s10965-017-1370-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-017-1370-9