Abstract
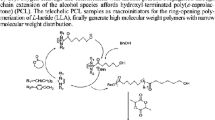
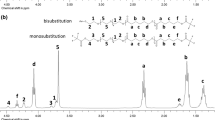
α,ω-Hydroxy telechelic poly(L-lactides) (HOPLLAOH) were synthesized by ring-opening polymerization of the L-lactide (L-LA) catalyzed by tin octoate [Sn(Oct)2] in the presence of two different types of initiators such as alkyl diols [HO−(CH2−CH2)m−OH] and ether diols [HO−(CH2−CH2−O)m−H] (where m = 2, 3, 4, 5, 6, and 8), and eventually, two different families of HOPLLAOH where the alkyl group (AG) [HO−PLLA−(CH2−CH2)m−PLLA−OH (HOPLLAaOH)] and ether group (EG) [HO−PLLA−(CH2−CH2−O)m−PLLA−OH (HOPLLAeOH)] are part of the backbone of the polymer with a systematic increase in these segments were synthesized. The number average molecular weight (M n) for all samples were similar [M n(NMR) = 1610–1980]. The weight percent (wt.%) of AG and EG had a dramatic effect on the glass transition temperature (T g) of HOPLLAaOH (from 19 to 3 °C) and HOPLLAeOH (from 19 to −1 °C), respectively, where the wt.% of AG or EG induced a decrease on the T g with a relationship that was inversely proportional. For poly(ester-urethanes) (PEUs) derived from HOPLLAaOH (PEUa) and HOPLLAeOH (PEUe) and 1,6-hexamethylene diisocyanate (HDI) showed an increase in the values of T g attributed to the hydrogen bonding of the urethanes groups in the main chain, for example from 19 (HOPLLA4aOH) to 39 °C (PEU4a) where 4a is indicating four aliphatic methylenes. However, the systematic variation in the repetitive unit from AG and EG in the PEUa and PEUe, respectively, it had also the similar effect on the T g, decreasing the values, for example, PEUa from 39 to 19 °C and PEUe from 39 to 17 °C, consistently as such as the previous HOPLLAOH species.











Similar content being viewed by others
References
Vink ETH, Rábago KR, Glassner DA, Springs B, O’Connor RP, Kolstand J, Gruber PR (2004) The sustainability of NatureWorks™ polylactide polymers and Ingeo™ polylactide fibers: an update of the future. Macromol Biosci 4:551–564
Janssen L, Moscicki L (2009) Thermoplastic starch, a green material for various industries. Wiley-VCH, Hong Kong,
Li S, Hu Y (2014) Poly(lactic acid) science and technology. In: Jimenez A, Peltzer M, Ruseckaite RA (eds) Processing, properties, additives, and applications. RSC, Oxford,
Báez JE (2010) How to obtain a degradable polymer in the laboratory: Synthesis of poly(D, L-lactide) and characterization. Educ quím 21:170–177
Si P, Luo F, Xue P, Yan D (2016) Crystallization mechanism, microstructure and mechanical property of poly(L-lactic acid) modified by introduction of hydrogen bonding. J. Polym Res 23:118–126
Kaur H, Rathore A, Raju S (2014) A study on ZnO nanoparticles catalyzed ring opening polymerization of L-lactide. J Polym Res 21:537–546
https://www.accessdata.fda.gov/scripts/fdcc/index.cfm?set=IndirectAdditives&id=STANNOUSETHYLHEXANOATE. Accessed 03 Mar 2017
Zhang X, Macdonald DA, Goosen MFA, Mcauley KB (1994) Mechanism of lactide polymerization in the presence of stannous octoate: the effect of hydroxy and carboxylic acid substances. J Polym Sci Part A Polym Chem 32:2965–2970
Sosnowski S, Lewinski P (2015) L-lactide polymerization catalyzed by tin(II) 2-ethyl-hexanoate. A deeper look at chain transfer reactions. Polym Chem 6:6292
Báez JE, Marcos-Fernández A, Galindo-Iranzo P (2011) Exploring the effect of the alkyl end group on poly(L-lactide) oligo-esters. Synthesis and characterization. J Polym Res 18:1137–1146
Choinska E, Muroya T, Swieszkowski W, Aoyagi T (2016) Influence of macromolecular structure of novel 2- and 4-armed polylactides on their physicochemical properties and in vitro degradation process. J Polym Res 23:1–11. https://doi.org/10.1007/s10965-016-1023-4
Báez JE, Galindo-Iranzo P, Marcos-Fernández A (2016) Poly(L-lactide) macrodiols (HOPLLAOH): influence of linear alkyl diols as initiators: Synthesis and characterization. Int J Polym Anal Charact 21:149–155
Monga S, Kaushik A, Gupta B (2016) Synthesis of L-lactide-based segmented polyurethanes. Polym Plast Technol Eng 55:943–948
Monga S, Kaushik A, Gupta B (2016) Optimization of process parameters for controlled ring-opening polymerization of lactide to produce poly(L-lactide) diols as precursors for polyurethanes. Polym Plast Technol Eng 55:1819–1830
Lazdina B, Stirna U, Tupureina V, Dzene A, Sevastyanova I (2006) Synthesis and properties of cross-linked poly(ester urethanes) from poly(lactide) triols and poly(caprolactone) diols. Proc Estonian Acad Sci Chem 55:85–92
Xue S, Pei D, Jiang W, Mu Y, Wan X (2016) A simple and fast formation of biodegradable poly(urethane-urea) hydrogel with high water content and good mechanical property. Polymer 99:340–348
Donvito A, Fantin G, Fogagnolo M, Giovannini PP, Scoponi M (2016) Novel tri- and tetrafunctional cholic acid-based initiators for the synthesis of star-shaped poly(L-lactide)s. Desig Mon Polym 19:535–544
Eldessouki M, Buschle-Diller G, Gowayed Y (2016) Solution-based synthesis of a four-arm star-shaped poly(L-lactide). Desig Mon Polym 19:180–192
Selukar BS, Parwe SP, Mohite KK, Garnaik B (2012) Synthesis and characterization of linear polylactic acid-based urethanes using Tin modified solid cloisite-30B catalyst. Adv Mat Lett 3:161–171
Ou B, Chen M, Huang R, Zhou H (2016) Preparation and application of novel biodegradable polyurethane copolymer. RSC Adv 6:47138–47144
Fabbri M, Soccio M, Costa M, Lotti N, Gazzano M, Siracusa V, Gamberini R, Rimini B, Munari A, Garcia-Fenandez L, Vazquez-Lasa B, San Roman J (2016) New fully bio-based PLLA triblock copoly(ester urethane)s as potential candidates for soft tissue engineering. Polym Degrad Stab 132:169–180
Lee SY, Valtchev P, Dehghani F (2012) Synthesis and purification of poly(L-lactic acid) using a one step benign process. Green Chem 14:1357–1366
Nakayama Y, Inaba T, Toda Y, Tanaka R, Cai Z, Shiono T, Shirahama H, Tsutsumi C (2013) Synthesis and properties of cationic ionomers from poly(ester-urethane)s based on polylactide. J Polym Sci Part A Polym Chem 51:4423–4428
Middleton H, Tempelaar S, Haddleton DM, Dove AP (2011) Organocatalytic synthesis of astaxanthin-containing poly(lactide)s. Polym Chem 2:595–600
Kricheldorf HR, Mix R, Weidner SM (2014) Poly(ester urethane)s derived from lactide, isosorbide, terephthalic acid, and various diisocyanates. J Polym Sci Part A, Polym Chem 52:867–875
Reddy KR, Raghu AV, Jeong HM (2008) Synthesis and characterization of novel polyurethanes based on 4,4′-{1,4-phenylenebis[methylylidenenitrilo]}diphenol. Polym Bull 60:609–616
Reddy KR, Raghu AV, Jeong HM, Siddaramaiah (2009) Synthesis and characterization of pyridine-based polyurethanes. Desig Mon Polym 12:109–118
Choi SH, Kim DH, Raghu AV, Reddy KR, Lee H-I, Yoon KS, Jeong HM, Kim BK (2012) Properties of graphene/waterborne polyurethane nanocomposites cast from colloidal dispersion mixtures. J Macromol Sci, Part B, Physics 51:197–207
Wang C, Zheng Y, Sun Y, Fan J, Qin Q, Zhao Z (2016) A novel biodegradable polyurethane based on poly(3-hydroxybutyrate-co-3-hydroxyvalerate) and poly(ethyleneglycol) as promising biomaterials with the improvement of mechanical properties and hemocompatibility. Polym Chem 7:6120–6132
Báez JE, Marcos-Fernández A (2015) A comparison of three different biodegradable aliphatic oligoesters (PGA, PLLA, and PCL) with similar linear alkyl end groups by DSC and SAXS. Int J Polym Anal Charact 20:637–644
Báez JE, Marcos-Fernández A (2011) A simple and rapid preparation of poly(glycolide) (PGA) oligomers catalyzed by decamolybdate anion in the presence of aliphatic alcohols. Int J Polym Anal Charact 16:269–276
Báez JE, Marcos-Fernández A, Galindo-Iranzo P (2011) On the effect of alkyl end group in poly(ε-caprolactone) oligomers: preparation and characterization. Polym Plast Technol Eng 50: 839–850
Báez JE, Marcos-Fernández A, Martínez-Richa A, Galindo-Iranzo P (2017) Poly(ε-caprolactone) diols (HOPCLOH) and their poly(ester-urethanes) (PEUs): The effect of linear aliphatic diols [HO-(CH2)m-OH] as initiators. Polym Plast Technol Eng 56: 889–898
Báez JE, Ramírez-Hernández A, Marcos-Fernández A (2010) Synthesis, characterization, and degradation of poly(ethylene-b-ε-caprolactone) diblock copolymer. Polym Adv Technol 21:55–64
Báez JE, Marcos-Fernández A (2012) Degradable poly (ester-ether urethane)s derived of AB2 miktoarm star copolymer poly (ethylene glycol-(ε-caprolactone)2) diol: synthesis, characterization and degradation. React Funct Polym 72:349–357
Báez JE, Zhao R, Shea KJ (2017) Synthesis of poly(methylene-b-ε-caprolactone) and poly(ε-caprolactone) with linear alkyl end groups: synthesis, characterization, phase behavior and compatibilization efficacy. Ind Eng Chem Res 56:10366–10383
Báez JE, Ramírez-Hernández A, Marcos-Fernández A (2011) The effect of trifluoroacetic anhydride on poly (ε-caprolactone) (PCL) oligomers. Int J Polym Anal Charact 16: 377–389
Zhong Z, Ankoné MJK, Dijkstra PJ, Birg C, Westerhausen M, Feijen J (2001) Calcium methoxide initiated ring-opening polymerization of ε-caprolactone and L-lactide. Polym Bull 46: 51–57
Ronda JC, Serra A, Mantecón A, Cádiz V (1994) End-group analysis of poly(phenyl glycidyl ether), 1 hydroxylic groups using 1H and 13C nuclear magnetic resonance. Macromol Chem Phys 195:3445–3457
Lemmouchi Y, Perry MC, Amass AJ, Chakraborty K, Schué F (2007) Novel synthesis of biodegradable poly(lactide-co-ethylene glycol) block copolymers. J Polym Sci Part A Polym Chem 45:2235–2245
McLain SJ, Drysdale DE (1992) Living ring-opening polymerization of ε-caprolactone by yttrium and lanthanide alkoxides. Polym. Prepr. (Am. Chem. Soc. Div. Polym. Chem.) 33: 174–175
Tong R (2017) New chemistry in functional aliphatic polyesters. Ind Eng Chem Res 56:4207–4219
Hamad K, Kaseem M, Yang HW, Deri F, Ko YG (2015) Properties and medical applications of polylactic acid: a review. Express Polym Lett 9:435–455
Kunal K, Robertson CG, Pawlus S, Hahn SF, Sokolov AP (2008) Role of chemical structure in fragility of polymers: a qualitative picture. Macromolecules 41:7232–7238
Ruan C, Wang Y, Zhang M, Luo Y, Fu C, Huang M, Sun J, Hu C (2012) Design, synthesis and characterization of novel biodegradable shape memory polymers based on poly(D,L-lactic acid) diol, hexamethylene diisocyanate and piperazine. Polym Int 61:524–530
Doppalapudi S, Jain A, Khan W, Domb AJ (2014) Biodegradable polymers-an overview. Polym Adv Technol 25:427–435
Arias V, Höglund A, Odelius K, Albertsson A-C (2014) Tuning the degradable profiles of poly(L-lactide)-based materials through miscibility. Biomacromolecules 15:391–402
Huang J, Xiong J, Liu J, Zhu W, Wang D (2013) Investigation of the In Vitro degradation of novel polylactide/nanohydroxyapatite composite for artificial bone. J Nanomaterials 2013: Article ID: 515741, ten pages
Hiltunen K, Seppälä JV, Itävaara M, Härkӧnen M (1997) The biodegradation of lactic acid-based poly(ester-urethanes). J Environ Polym Degrad 5:167–173
Acknowledgements
José E. Báez thanks the “Programa para el Desarrollo Profesional Docente (PRODEP, Protocolo 166155)”, Dirección de Apoyo a la Investigación y al Posgrado (DAIP) at University of Guanajuato (UG), and “Sistema Nacional de Investigadores (SNI)” in México for financial support of the work. José E. Báez also thanks to Ángel Marcos-Fernández for believing in these ideas and providing financial support for the reagents. José E. Báez also thanks to the UG for the recent opportunity to work as an Assistant Professor. Marvin was used for drawing, displaying, and characterizing chemical structures, substructures, and reactions (Marvin Sketch 6.1.3, 2013, ChemAxon; http://www.chemaxon.com); a free software program with an academic license was provided by ChemAxon. Ángel Marcos-Fernández would like to thank the Ministry of Economy and Competitiveness (MINECO) for the financial support of this work within the framework of the Plan Nacional de I + D + I through the research project MAT2014-52644-R. Finally, José E. Báez thanks to Gema Reina Mendieta and David Gómez Varga for the acquisition of the NMR spectra and SEM micrographs, respectively.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Báez, J.E., Marcos-Fernández, Á., Navarro, R. et al. Study on the effect of linear alkyl [CH2CH2]m and ether [CH2CH2O]m groups in the α,ω-hydroxy telechelic poly(L-lactide) (HOPLLAOH) and their poly(ester-urethanes) (PEUs). Synthesis and Characterization. J Polym Res 24, 199 (2017). https://doi.org/10.1007/s10965-017-1364-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-017-1364-7