Abstract
The objective of this study was to analyse how macromolecular structure of polylactides influences their properties and degradation rate. To achieve this, novel 2- and 4-armed PDLLA and PLLA (noted as 2b and 4b) were synthesized by ring-opening method. 1,4-butanediol and pentaerythritol were used as initiators and stannous octoate was used as catalyst. The obtained polymers were investigated in terms of molecular weight by size exclusion chromatography, thermal properties by differential scanning calorimetry and thermogravimetry, and hydrophilicity by the contact angle measurements. The in vitro degradation test was carried out in PBS solution at 37 °C by means of the mass loss, water uptake, molecular weight and thermal properties changes. The branched polylactides including 2bPDLLA, 4bPDLLA, 2bPLLA and 4bPLLA were successfully synthesized and the average molecular weights were around 40-45 kDa. The numbers of arms in each polymer just slightly influenced the thermal properties and the contact angle. The crystallinity of 4bPLLA was 23 %, whereas for 2bPLLA it was 41 %. The degradation rates of both 2b and 4bPLLA were similar and the degradation process was similar only during first 7 weeks. After this period, the degradation rate of 4bPDLLA increased. Consequently, thermal properties and degradation profiles of the branched polymers would depend on plural factors, such as chain length and crystallinity in branched structure.
Similar content being viewed by others
Introduction
Biodegradable synthetic polymers have been extensively studied throughout last few decades. Their key advantages are ability to tailor mechanical properties and degradation kinetics. They can be fabricated into various desired shapes and designed with chemical functional groups. Among them are aliphatic polyesters, such as polyglicolide, polylactide and their copolymers, polycaprolactone or polydioxanone, which have attracted considerable attention because of their biocompatibility [1–3]. Moreover, these polymers degrade by hydrolysis of ester bonds and degradation products are resorbed through the metabolic pathways [4].
One of the most common aliphatic polyesters used in biomedical application is polylactide (PLA). The basic building block for PLA is lactic acid with an asymmetric carbon atom and exist in two optically active configuration: L(+) and D(−). Optically pure poly(L-lactide) PLLA and poly(D-lactide) PDLA are crystalline with a glass-transition and melt temperature of about 55 °C and 175 °C, respectively, whereas poly(D,L-lactide) PDLLA is amorphous with glass transition temperature slightly lower than for optically pure PLLA and PDLA [5].
Despite wide use of PLAs, there are some limitations of physicochemical properties, such as lack of controlled degradation based on its high crystallinity. It is known that molecular weight, crystallinity and samples dimensions are the most important parameters which influence the degradation rate, however hydrophilicity, permeability and glass transition temperature can also affect this process [6–8]. In order to better control degradation of PLAs, many modifications were done, mainly based on linear copolymerization or block copolymerization [9, 10].
Almost twenty years ago, our research group proposed that polymer architecture would also considerably affect the thermal properties of typical aliphatic polyester, poly(ε-caprolactone) (abbreviated as PCL) [11]. Actually, PCL is well-known semi-crystalline polymer and it has melting point around 60 °C. We intended to design new temperature responsive materials using PCL, therefore, that temperature is too high to consider the biomedical application. It was critical to modulate the melting point near body temperature. Then we designed branched PCL to control crystallinity of PCL. As expected, the combination of two kinds of the branched PCL, that were 2-armed and 4-armed PCL, precisely controlled the softening point of their cross-linked materials near body temperature [12].
So far, there are not many papers describing the influence of polylactides architecture on their properties. Korhonen et al. have synthesized and characterized star-shaped PLAs with 1, 2, 4, 6 and 10 arms [13]. Srisa-ard et al. have analysed influences of arm numbers (1, 4, 6 and 16) and length on the thermal properties [14]. Hao et al. have investigated the 1, 3, 4, 5 and 6-armed polymers in the terms of crystallization kinetics [15]. Tsuji et al. have also analysed the crystallization behaviour of PLAs, but only 1 – and 2-arm linear PLLA [16]. They also investigated the hydrolytic degradation and thermal properties of 1 – and 2-arm PDLLA [17]. Degradation was also investigated by Yuan et al. [18], but he has studied only linear and hexa-armed PLLA and PDLLA.
In this paper the 2- and 4-armed PLLA and PDLLA was investigated. It is complementary study to the mentioned above. The new materials were synthesized and characterized to show how their new architecture influences their physic-chemical properties and degradation rate.
Materials and methods
Materials
L-lactide and D,L-lactide were kindly supplied by the Musashino Chemical Laboratory (Japan) and recrystallized twice from ethyl acetate before use. 1,4-butanediol (>99 %) and pentaerythritol (>98 %) were purchased from Tokyo Chemical Industries (Japan). Tin 2-ethylhexanoate (95 %) and phosphate buffer saline tablets pH = 7.4 were from Sigma Aldrich (Japan and Poland, respectively). All solvents (Wako Pure Chemical Industries, Japan) were analytical grade; only amylene-stabilized chloroform (POCH, Poland) was HPLC grade. All chemicals were used as received.
Synthesis of 2- and 4-armed polylactides
The branched (2- and 4-armed) poly(L-lactide) PLLA and poly(D,L-lactide) PDLLA were synthesized by ring-opening polymerization (ROP) in bulk under nitrogen atmosphere. The 0.25 mol (36 g) of monomer (L-lactide or D,L-lactide) and bi- or tetra-functional initiator were placed in round-bottomed flask and dried under reduced pressure for 6 h. The molar ratio of initiator to monomer was 1:320. Then a catalyst was added and flask was filled with nitrogen. The polymerization was carried out for 24 h at 140 °C (for PDLLA) or 120 °C (for PLLA) in silicon oil bath using the magnetic stirrer. After reaction polymer was dissolved in 400 ml tetrahydrofuran and precipitated into 1500 ml mixed solvent (1:1) of n-hexane and diethyl ether. Precipitated polymers were dried under vacuum for 24 h. The reaction of polymerization is shown in Fig. 1. Four types of polymers were obtained: 2-armed PDLLA, 2-armed PLLA, 4-armed PDLLA and 4-armed PLLA noted 2bPDLLA, 2bPLLA, 4bPDLLA and 4bPLLA, respectively.
Polymers characterization
Fourier transform infrared spectroscopy
Chemical structure of synthesized polylactides was analysed by ATR-FTIR (attenuated total reflectance - Fourier Transform Infrared Spectroscopy). For each sample 64 scans in the range of wavenumbers 400–4000 cm−1 with resolution of 4 cm−1 were done and averaged.
Molecular weight measurements
The molecular weights of the polymers were measured by size exclusion chromatography and multi-angle laser light scattering (SEC-MALLS). This method doesn’t require the narrow distributed polymer standards for calibration and is more suitable for star-shaped or branched polymers [19, 20]. The number and weight average molecular weights (Mn and Mw) were determined by modular system Agilent 1200 series HPLC with refractive index detector and 3-angle (48°, 90° and 132°) laser light scattering detector MiniDAWN TREOS (Wyatt Technology). All samples (2 mg/ml) were filtered through PTFE 0.2 μm membrane before analysis to remove the small amount of dust. Measurements were made at 30 °C with PLgel 5 μm MIXED-C column (300 × 7.5 mm). The chloroform was used as the solvent at flow rate of 1 ml/min. The evaluation of the results was made using ASTRA 5.3 software (Wyatt Technology). The specific refractive index PLA in chloroform dn/dc = 0.024 mL/g [21] was used for calculations.
Thermal properties
The thermal properties were analysed using differential scanning calorimeter (DSC) Q200 TA Instruments. Samples of about 6 mg were analysed in alumina pans. The measurements were run according to heat-cool-heat procedure from 0 °C to 200 °C for PLLA and from 0 °C to 80 °C for PDLLA, at heating and cooling rate of 10 °C/min. In order to eliminate the internal stresses the glass transition temperature Tg, melting temperature Tm, crystallization temperature Tc and crystallinity Xc were determined from second heating scan.
Thermal stability of polymers was analyzed using thermogravimetric analyzer Q5000 TA Instruments. The mass of each specimen was 8-10 mg and the reaction environment was flowing nitrogen (25 ml/min). The TG curves were recorded in the temperature range from 50 °C to 450 °C, at heating rate of 10 °C/min. The DSC and TGA results were analyzed in Universal Analysis 2000 software (TA Instruments).
X-ray diffraction
The X-ray diffraction (XRD) analysis for PLLA samples was carried out with using Bruker AXS D8 DISCOVER diffractometer using Cu Kα (0.1542 nm) radiation. The scans were made on PLLA casted films in the 2θ range of 14–26°.
Surface wettability
The wettability of the polymers surface was evaluated on the basis of water static contact angle. The measurements were made using a contact angle goniometer OCA20 (Dataphysics). The samples were prepared as thin coatings on the microscope glass slides by dip-coating method. After completely dried, 3 μl of water droplet was dropped on the airside surface of the film at room temperature, and the contact angle was immediately measured. 10 measurements were made for each sample and the results were averaged.
In vitro hydrolytic degradation
In vitro degradation was carried out in phosphate buffered saline (PBS) pH = 7.4 at 37 °C. Degradation medium was changed every week. Polymers were prepared by wet cast technique as follows. Polymers were dissolved in chloroform and poured into Petri dishes and left for drying under chemical hood for 24 h. Then films were dried up in vacuum dryer for one week (50 mbar, 25 °C). Three samples of 10x15mm2 and thickness of 0.35 um for each time point were cut and immersed in 7 ml of PBS. The mass loss and water uptake were calculated gravimetrically, molecular weight and thermal properties changes were analyzed by SEC and DSC according to the procedures described previously. Degraded samples were investigated also by ATR-FTIR and SEM observations. SEM images were performed with using Phenom ProX (PhenomWorld). Samples for microscopic analysis were sputtered with 7 nm gold layer.
Statistical analysis
The results were evaluated statistically by t-Test - paired comparison of means (KyPlot 2.0 beta 13 software). The comparison of 2b and 4b forms of PLAs, not PDLLA and PLLA was done. Data are expressed as a mean ± standard deviation (SD). Three significance levels were used: *p < 0.05, **p < 0.01, ***p < 0.001.
Result and discussion
Polymers synthesis and characterisation
The 2- and 4-armed PLAs were prepared by using bi- and tetra-functional initiators. The very common 1,4-butanediol and pentaerythritol were used [13, 22, 23]. The tin 2-ethylhexanoate (stannous octoate) was chosen as catalyst because of its solubility in lactones, low toxicity, FDA approval, high catalytic activity, and ability to give high-molecular-weight polymers with low racemization [24]. The synthesized polymers had narrow molar mass distribution (Table 1). The Mw/Mn was in the range 1.1–1.3. Such low polydispersity has proved the high efficiency of polymerization and good quality of purification process.
The purity of synthetized polymers was also confirmed by ATR-FTIR spectra analysis (Fig. 2). There was not any peak at 935 cm−1, which is characteristic for unreacted monomer of lactide [25]. Characteristic peaks were observed for the same wavenumbers for 2b and 4b PLAs. It means, that the molecular architecture did not change the FTIR spectra of polylactides. Only one difference was observed. For PLLAs additional peak at 920 cm−1 was recorded. This peak is characteristic for crystalline phase [26, 27]. According to Vasanthan and Ly [27] the band ratio of 920 cm−1 and 957 cm−1 is well correlated with the crystallinity obtained from DSC. For our polymers the A920/A957 ratios were 0.69 and 0.72 for 4bPLLA and 2bPLLA, respectively. These results suggest, that linear PLLA contains more crystalline phase than the 4-armed PLLA.
Thermal properties like glass transition temperature, melting temperature and crystallinity were obtained from DSC thermograms and are summarized in Table 1. The degree of crystallinity Xc was calculated according to Eq. 1, where ΔHm is the melting enthalpy, ΔHc is the crystallization enthalpy and 93.6 J/g is the melting enthalpy of totally crystalline PLLA sample [28].
Obtained results are in agreement with ATR-FTIR spectra analysis. Significantly higher degree of crystallinity was calculated for 2b-poly(L-lactide) than for star-shaped form. Longer polymer chains can easier form crystals, than shorter ones characteristic for 4-armed form of PLLA.
The Tg of 4-armed polymers, both PLLA and PDLLA, is lower than for 2-armed. These results are in agree with the Flory–Fox equation:
where: \( {T}_g^{\mathit{\infty}} \)is the Tg at the infinite molecular weight, K is a constant representing the excess free volume of the end groups for polymer chains, and Mn is the number average molecular weight. 4b PLAs have higher value of K parameter than 2b PLAs, therefore their Tg are lower. This effect is also related to the lower chain mobility and stronger inter-chain interaction of 2-armed PLAs. It is known, that longer polymer chains are less mobile that shorter one. This can affect the diffusion of water into polymer, accompanied with changing the degradation rate of the material. These results are also in agree with data presented by Sarisa-ard [29] and Tsuji [17]. The melting temperature (Tm) of 2bPLLA is a bit higher than Tm of 4bPLLA. The same dependence was observed by Yuan et al. [18] and Hao et al. [15].
The thermogravimetric curves are shown in Fig. 3. They show that 4-armed PLAs have slightly lower thermal stability. The onset of decomposition (Tonset) was 275.8 °C and 277.4 °C for 4bPDLLA and 2bPDLLA, respectively. For 4bPLLA and 2bPLLA it was 277.6 °C and 280.6 °C, respectively. All of them undergo one-stage thermal decomposition. The maximum decomposition rate was observed at temperature 307.3 °C and 308.0 °C for 4- and 2-armed PDLLA, whereas it was 293.9 °C and 298.7 °C for 4- and 2-armed PLLA. Sarisa-ard [29] and Yuan [18] attribute these differences to shorter polymer chains and higher content of thermally unstable hydroxyl groups in 4-armed PLAs. It can be also related to the crystalline structure. Longer arms of 2bPLLA are capable to create perfect crystal lattices, which create more dense structure and larger crystals than 4bPLLA. Thus, they can exhibit higher values of Tm and better thermal stability. This theory was confirmed by XRD analysis (Fig. 4). For 2bPLLA the two strongest reflection are located at 16.9° and 19.2°. Two smaller peaks at 14.9° and 22.6° were observed. This is typical pattern for α PLLA. The slight shifts of the peaks to the lower values of 2θ, to 16.86°, 19.23° and 22.45°, respectively, were observed for 4bPLLA. That corresponding to the increase of lattice spacing characteristic for α’ crystalline structure [30–32]. However, for 2bPLLA was observed additional small peak at 24.3°, which is also characteristic for α’ phase. Therefore, the most probable is coexistence of these two crystalline forms. This statement is also in agreement with DSC measurements. Both, 2- and 4-armed PLLA, crystallize in the rage 100–120 °C, which is the critical temperature region for α’ and α-form crystals formation, where the mixture of both this form coexists. Therefore, the differences in thermal properties are probably caused by different ratio of α’ and α forms crystals for 4b and 2b PLLA.
From the point of view of the hydrolytic degradation process aiming at biomedical application, very important property is hydrophilicity of polymer surface. It is known, that non-modified PLLA is more hydrophobic than PDLLA. This is an effect of chirality [33]. The effect of molecular architecture on hydrophilicity was analysed in this study. The images of water droplets on the surface of 2- and 4-armed PLAs with racemic or L-form sequences are shown in Fig. 5. The average value of water static contact angle was 71.9 ± 1.4° and 73.9 ± 1.3° for 4bPDLLA and 2bPDLLA, respectively. For 4bPLLA and 2bPLLA it was 95.8 ± 4° and 94.9 ± 5.3°, respectively. In both cases, no statistical difference was observed, therefore, the number of arms didn’t affect the surface hydrophilicity. Taking this into account, the number of arms of polymer seems to be not suitable parameter to control the hydrophilicity or hydrophobicity of polymer surface. Better results would be obtained by introducing some hydrophilic groups, segments or whole arms into star-shaped structures [14, 34].
In vitro hydrolytic degradation
The process of hydrolytic degradation of polymeric materials proceeds in few steps. At the beginning water diffuses into the polymer and initiates hydrolytic reaction. The short polymer chains with acidic end-groups are formed and autocatalyse the reaction. When the critical molecular weight is reached the oligomers start to diffuse out from the polymer matrix and that results in mass loss. The water molecules diffuse into the voids created by the removed oligomers and on the one hand increase hydrolysis, on the other hand encourage oligomers diffusion [35]. Because of this complexity of the process, a few parameters, as mass loss, water uptake and molecular changes should be monitored during degradation. In this study the mass loss and water uptake were calculated using the equations (3) and (4), respectively, where m0 is the initial dry mass of the sample and mt is the final dry mass of the sample and mt(wet) is the weight of polymer sample retrieved from PBS.
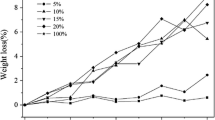
Although having similar original molecular weight the investigated polylactides have shown differences in weight loss during degradation tests (Fig. 6 a, b). The relationship between macromolecular structure and mass loss was faster observed for amorphous PDLLAs (5th week of degradation), than for semicrystalline PLLAs (15th week of hydrolysis). That indicates their different abilities of degradation. It is known that PDLLAs degrade faster than PLLAs, what can be attributed to the amorphous structure of PDLLA. As it is known, hydrolytic degradation starts from amorphous region. Moreover, the low water uptake of PLLAs (Fig. 6 d), caused by their hydrophobicity, could make more difficult the diffusion of the oligomers from the polymer. For both, PDLLA and PLLA, higher weight loss for 4-armed PLAs was observed. The same relation was obtained by Tsuji et al. [17], Yuan et al. [18] and Cai et al. [34]. They ascribe it to the much shorter PLA chains in the star-shape polymer. Shorter PLA segments would have more affinity suitable to enhance the hydrolysis. On the other word, if the polymer chains are short enough, the bulk degradation mechanism of aliphatic polyester (accompanying with slow weight loss) could change into surface degradation (with faster weight loss) [36].
According to the study of de Jong et al. [37], OH groups of chain ends of the lactic acid oligomers play a crucial role in the degradation in alkaline and acidic medium. Therefore, the higher degradation rate via the chain-end scission of 4-armed PLAs was expected. However, the analysis of molecular weight changes (Fig. 7) has not confirmed that. Despite different arms number the similar Mw decrease was observed. It suggests that effect described by de Jong et al. could be counterbalanced by higher chain mobility and weaker inter-chain interaction of 2-armed PLAs [17] and by the random polylactide chain cleavage. The second mechanism seems to play the crucial role, because both PLAs have shown the tendency to faster molecular weight loss for 2b macromolecules, than for star-shape ones.
The thermal analysis of degraded samples after 11 and 19 weeks for PDLLAa and PLLAs, respectively, has shown that the glass transition and melting temperatures decrease with increase of the degradation time. The Tg decreases from 38.5 °C to 31.4 °C for 2bPDLLA, and from 37.7 °C to 29 °C for 4bPDLLA (Fig. 8 a). This decrease of characteristic temperatures is known and described in literature [38, 39]. This is an effect of decreasing of molecular weight, presence of residual monomers, oligomers and water molecules in polymers, which act as plasticizers. On the other hand, in terms of 2bPLLA and 4bPLLA, only slight changes were observed (Fig. 8 b). The Tg decreases from 60.9 °C to 59.8 °C for 2bPLLA, and from 60.6 °C to 59.0 °C for 4bPLLA. So small changes could be an effect of not so considerable degradation rate. After longer degradation period, more than 19 weeks, the results similar to 2bPDLLA and 4bPDLLA would be obtained even in 2bPLLA and 4bPLLA. Another effects of a degradation process are decrease of melting temperature, occur of double melting peak and increase of crystallinity, observed for PLLA. Melting temperature decreases from 173.5 °C to 172.7 °C for 2bPLLA and from 171 °C to 169.8 °C for 4bPLLA. Decrease of Tm is an effect of lowering of molecular weight and wide distribution of polymer chain. The Xc increase from 41.3 % to 57.9 % and from 22.9 % to 33.8 % for 2bPLLA and 4bPLLA, respectively. The crystallinity of poly(L-lactides) increased, because of degradation process, which starts from amorphous regions, but also because of macromolecules reorganisation. Water, which diffuses into polymer matrix, acts as plasticiser and facilitates molecular chain movement and organisation. The double melting peaks observed at DSC curves of degraded samples were caused by degradation, but also cold crystallization process and coexistence of two crystalline forms [40].
After degradation process changes in surface of polymer samples were also observed. For both PLAs the samples after 11 weeks of incubation were analysed. After 19 weeks of degradation PLLAs samples became very brittle and their preparation for SEM observation was practically impossible, because damages made during sample mounting overlapped changes caused by hydrolysis process. In Fig. 9 SEM images of samples before and after degradation are presented.
The amorphous PDLLA samples characterise more degraded structure. There are visible developed, porous surface of the samples. In the case of semi-crystalline PLLA the changes are smaller. After degradation crystallites become more clear, especially in star-shaped poly(L-lactide). It is an effect of degradation mainly amorphous phase of polymers and superficial character of the degradation process. At SEM images of the samples after degradation smaller crystals for 4bPLLA were observed. That confirmed mentioned previously statement, that bigger crystallites are formed by linear polymer than by 4-armed one. For both polymers, amorphous PDLLA and semi-crystalline PLLA, bigger changes were observed for samples with 4-armed macromolecular architecture, what is in agreement with other degradation results.
Hydrolytic degradation belongs to the class of chemical degradation. The mechanism is associated with hydrolysis of ester linkage (Fig. 10). As a result of this reaction the content of C-O-C bonds decrease, whereas amount of C = O bonds stays at the same level. In our study it was analysed by ATR-FTIR spectra analysis.
The comparison of the degradation rate was done on the basis of analysis the ratio of absorbance of peak characteristic for vibration of C-O-C bonds presented at 1081 cm−1 to absorbance of peak characteristic for vibration of C = O bonds observed at 1743 cm−1(AvCOC/AvC=C). For 4bPDLLA the ratio was equal 1.05 and 0.79, whereas for 2bPDLLA it was 1.02 and 0.82, before and after degradation, respectively. The results have shown, that 4bPDLLA degraded faster than linear PDLLA. Similar dependence was observed also for PLLAs. The AvCOC/AvC=C ratio decrease from 1.17 to 1.15 for 4bPLLA and from 1.20 to 1.19 for 2bPLLA. These small changes is an effect of low degradation rate of semi-crystalline polylactides. These results are very well correlated with the molecular weight changes, which were bigger for amorphous PDLLAs than for PLLAs.
Practically all described and analysed above properties of 2- and 4-armed polylactides are strongly dependent on molecular weight. Therefore, during synthesis the assumption of preparing polymers with similar molar masses, about 40 kDa, which is typical for drug carriers, our future goal. This allowed to eliminate one important variable from analysis. On the other hand, it caused differences in the length of polymer arm, what in our opinion caused differences in properties between linear and star-shaped polylactides. As it was shown by Xie et al. [41], above some critical arms molecular weight, for polycaprolactones it was 3400 Da, properties of polymers, especially thermal properties and crystallinity, are strongly dependent on arm length, not on the core. It happens, because the significant influence of the central part of macromolecule on arms chains mobility, and properties connected with this effect, is limited only to relatively short arms. Similar relationship is expected also for PLAs. In this manuscript the shortest arm was close to 20,000 Da, therefore the influence of a core structure on the PLAs properties is much lower than the influence of arms chain length.
Conclusions
In the present study novel 2- and 4-armed PDLLA and PLLA were synthesized and characterized. The effect of molecules architecture of the novel polylactides on their properties and degradation were analysed. Due to weaker inter-chain interaction, the 4-armed PLAs have slightly lower Tg, and insignificantly lower thermal stability. The higher crystallinity of 2bPLLA was also observed. There was small difference in the hydrophilicity of 2b and 4b PLAs. The hydrolytic degradation was slightly faster for polylactides of more complex architecture (4bPLAs). This is an effect of few aspects like shorter arm’s length or easier oligomers leaching. It can be also affected by higher content of end-chain hydroxyl groups in 4bPLAs. Moreover, thanks to changing of macromolecular structure also water uptake can be controlled.
All properties mentioned above are very important, especially in the case of biodegradable polymers for biomedical application. The presented results have shown the potential of designing polylactides properties by using different star-shaped architecture.
References
Sliedregt A, Radder AM, Groot K, Blitterswijk CA (1992) In vitro biocompatibility testing of polylactides part I proliferation of different cell types. Journal of Materials Science: Materials in Medicine 3:365–370
Zamiri P, Kuang Y, Sharma U, Ng TF, Busold RH, Rago AP, et al. (2010) The biocompatibility of rapidly degrading polymeric stents in porcine carotid arteries. Biomaterials 31:7847–7855
Serrano MC, Pagani R, Vallet-Regi M, Peña J, Ramila A, Izguierdo I, et al. (2004) In vitro biocompatibility assessment of poly(ε-caprolactone) films using L929 mouse fibroblasts. Biomaterials 25:5603–5611
Kulkarni RK, Pani KC, Neuman C, Leonard F (1966) Polylactic acid for surgical implants. Archives of Surgery 93:839–843
Ahmed J, Zhang J, Song Z, Varshney S (2009) Thermal properties of polylactides. Journal of Thermal Analysis and Calorimetry 95:957–964
Grizzi I, Garreau H, Li S, Vert M (1995) Hydrolytic degradation of devices based on poly(dl-lactic acid) size-dependence. Biomaterials 16:305–311
Schliecker G, Schmidt C, Fuchs S, Wombacher R, Kissel T (2003) Hydrolytic degradation of poly(lactide-co-glycolide) films: effect of oligomers on degradation rate and crystallinity. International Journal of Pharmaceutics 266:39–49
Alexis F (2005) Factors affecting the degradation and drug-release mechanism of poly(lactic acid) and poly[(lactic acid)-co-(glycolic acid)]. Polymer International 54:36–46
Zhou S, Deng X, Li X, Jia W, Liu L (2004) Synthesis and characterization of biodegradable low molecular weight aliphatic polyesters and their use in protein-delivery systems. Journal of Applied Polymer Science. 91:1848–1856
Li SM, Rashkov I, Espartero JL, Manolova N, Vert M (1996) Synthesis, characterization, and hydrolytic degradation of PLA/PEO/PLA triblock copolymers with long poly(l-lactic acid) blocks. Macromolecules 29:57–62
Takao A, Fusae M, Yu N (1994) Preparation of cross-linked aliphatic polyester and application to thermo-responsive material. Journal of Controlled Release 32:87–96
Uto K, Yamamoto K, Hirase S, Aoyagi T (2006) Temperature-responsive cross-linked poly(ε-caprolactone) membrane that functions near body temperature. Journal of Controlled Release. 110:408–413
Korhonen H, Helminen A, Seppälä JV (2001) Synthesis of polylactides in the presence of co-initiators with different numbers of hydroxyl groups. Polymer 42:7541–7549
Srisa-ard M, Baimark Y (2011) Water-soluble stimuli responsive star-shaped segmented macromolecules. Polymers 3:1911–1933
Hao Q, Li F, Li Q, Li Y, Jia L, Yang J, et al. (2005) Preparation and crystallization kinetics of new structurally well-defined star-shaped biodegradable poly(l-lactide)s initiated with diverse natural sugar alcohols. Biomacromolecules 6:2236–2247
Tsuji H, Sugiura Y, Sakamoto Y, Bouapao L, Itsuno S (2008) Crystallization behavior of linear 1-arm and 2-arm poly(l-lactide)s: effects of coinitiators. Polymer 49:1385–1397
Tsuji H, Yamamoto J (2011) Hydrolytic degradation and thermal properties of linear 1-arm and 2-arm poly(dl-lactic acid)s: effects of coinitiator-induced molecular structural difference. Polymer Degradation and Stability. 96:2229–2236
Yuan W, Zhu L, Huang X, Zheng S, Tang X (2005) Synthesis, characterization and degradation of hexa-armed star-shaped poly(l-lactide)s and poly(d,l-lactide)s initiated with hydroxyl-terminated cyclotriphosphazene. Polym Degrad Stab. 87:503–509
Thijs HML, Meier MAR (2007) Schubert US. Application possibilities of preparative size exclusion chromatography to analytical problems in polymer science e-Polymers 046:1–10
Ahn S, Lee H, Lee S, Chang T (2012) Characterization of branched polymers by comprehensive two-dimensional liquid chromatography with triple detection. Macromolecules 45:3550–3556
Malmgren T, Mays J, Pyda M (2006) Characterization of poly(lactic acid) by size exclusion chromatography, differential refractometry, light scattering and thermal analysis. Journal of Thermal Analysis and Calorimetry. 83:35–40
Muroya T, Yamamoto K, Aoyagi T (2009) Degradation of cross-linked aliphatic polyester composed of poly(ε-caprolactone-co-d,l-lactide) depending on the thermal properties. Polym Degrad Stab 94:285–290
Hakala RA, Korhonen H, Holappa S, Seppälä JV (2009) Hydrophobicities of poly(ε-caprolactone) oligomers functionalized with different succinic anhydrides. European Polymer Journal. 45:557–564
Kricheldorf HR, Serra A (1985) Polylactones. Polymer Bulletin 14:497–502
Braun B, Dorgan JR, Dec SF (2006) Infrared spectroscopic determination of lactide concentration in polylactide: an improved methodology. Macromolecules 39:9302–9310
Kang S, Hsu SL, Stidham HD, Smith PB, Leugers MA, Yang X (2001) A spectroscopic analysis of poly(lactic acid) structure. Macromolecules 34:4542–4548
Vasanthan N, Ly O (2009) Effect of microstructure on hydrolytic degradation studies of poly (l-lactic acid) by FTIR spectroscopy and differential scanning calorimetry. Polymer Degradation and Stability. 94:1364–1372
Fischer EW, Sterzel HJ, Wegner G (1973) Investigation of the structure of solution grown crystals of lactide copolymers by means of chemical reactions. Colloid & Polymer Science 251:980–990
Srisa-ard M, Baimark Y (2010) Effects of Arm Number and Arm Length on Thermal Properties of Linear and Star-shaped Poly(D,L-lactide)s. Am J Appl Sci 10:1937–1943
Pan P, Kai W, Zhu B, Dong T, Inoue Y (2007) Polymorphous crystallization and multiple melting behavior of poly(l-lactide): molecular weight dependence. Macromolecules 40:6898–6905
Pan P, Zhu B, Kai W, Dong T, Inoue Y (2008) Effect of crystallization temperature on crystal modifications and crystallization kinetics of poly(L-lactide). Journal of Applied Polymer Science 107:54–62
Di Lorenzo ML, Cocca M, Malinconico M (2011) Crystal polymorphism of poly(l-lactic acid) and its influence on thermal properties. Thermochimica Acta 522:110–117
Yi Q, Wen X, Li L, He B, Nie Y, Wu Y, et al. (2009) The chiral effects on the responses of osteoblastic cells to the polymeric substrates. European Polymer Journal 45:1970–1978
Cai Q, Zhao Y, Bei J, Xi F, Wang S (2003) Synthesis and properties of star-shaped polylactide attached to poly(Amidoamine) dendrimer. Biomacromolecules 4:828–834
Engineer C, Parkih J, Raval A (2011) Review on hydrolytic degradation behavior of biodegradable polymers from controlled drug delivery system. Trends in Biomaterials and Artificial Organs 25:79–85
Breitenbach A, Pistel KF, Kissel T (2000) Biodegradable comb polyesters. Part II. Erosion and release properties of poly(vinyl alcohol)-g-poly(lactic-co-glycolic acid). Polymer 41:4781–4792
de Jong SJ, Arias ER, Rijkers DTS, van Nostrum CF (2001) Kettenes-van den Bosch JJ, Hennink WE. New insights into the hydrolytic degradation of poly(lactic acid): participation of the alcohol terminus. Polymer 42:2795–2802
Giardino R, Fini M, Aldini N, Giavaresi G, Rocca M, Orienti L, et al. (1997) Experimental evaluation of a resorbable intramedullary plug for cemented total hip replacement. Biomaterials 18:907–913
Saha SK, Tsuji H (2006) Hydrolytic degradation of amorphous films of L-lactide copolymers with glycolide and D-lactide. Macromolecular Materials and Engineering 291:357–368
Zhang J, Tashiro K, Tsuji H, Domb AJ (2008) Disorder-to-order phase transition and multiple melting behavior of poly(l-lactide) investigated by simultaneous measurements of WAXD and DSC. Macromolecules 41:1352–1357
Xie W, Jiang N, Gan Z (2008) Effects of multi-arm structure on crystallization and biodegradation of star-shaped poly(ε-caprolactone). Macromolecular Bioscience 8:775–784
Acknowledgments
This work was financially supported by WUT-NIMS Joint Graduate School Program, European Regional Development Fund within the Innovative Operational Program in the frame of project BIO-IMPLANT (Grant No. POIG.01.01.02-00-022/09) and National Centre for Research and Development in the frame of project MentorEye (STRATEGMED1/2333624/4/NCBR/2014).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Choinska, E., Muroya, T., Swieszkowski, W. et al. Influence of macromolecular structure of novel 2- and 4-armed polylactides on their physicochemical properties and in vitro degradation process. J Polym Res 23, 132 (2016). https://doi.org/10.1007/s10965-016-1023-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-016-1023-4