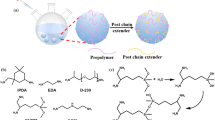
Abstract
Two solvent-free processes without using volatile organic chemical (VOC) for making polyurea elastomers have been successfully developed from diamines and diphenyl carbonate (DPC) to meet stringent public demands for a high standard green alternative Anastas and Eghbali (Chem Soc Rev 39 (1):301–312, 2010). In this new non-isocyanate route (NIR) which is an improvement over our previous process to polyurea elastomers, molten DPC are utilized as the carbonylation agents, where DPC reacts sequentially with 1,6-hexanediamine (HDA), polyether diamines of 2000 molecular weight and short chain diamines such as isophorondiamine (IPDA) or 4,4′-diaminodicyclohexylmethane (H12MDA). No solvent was added in the synthesis of these new non-solvent polyurea. Alternatively, the above process was modified with an 3 % addition of a water dispersant such as 3-[(2-aminoethyl)amino]-1-propane sulfonic acid sodium salt (ESA) to make waterborne polyurea elastomers to facilitate the mixing in the preparation and in the film-making steps for the final products. In both solvent-free processes, the long-chained polyether diamine and the phenol generated from the displacement reaction of diphenyl carbonate were utilized as diluents to lower the viscosities of the reaction mixtures. Unexpectedly, ultra high molecular weight products were resulted particularly for the waterborne polyurea products measuring in excess of 1,000,000 g/mol in some cases. All samples were characterized by FT-IR, H-NMR, TGA, DSC, AFM and GPC. Since the polymers were prepared under mild conditions in absence of a VOC solvent, the new products were found to mostly exist in highly phase-segregated states, exhibiting high heat-resistant temperatures with Td(5%) of well over 300 °C with high elongation (>600 %) for the optimized products. Thus, this solvent-free syntheses and environment-friendly polyurea products should find usefulness in many practical applications.























Similar content being viewed by others
References
Primeaux II DJ (2004) The Inspection of Coatings and Linings 2nd Edition. Polyurea Elastomer Technology: Histroy, Chemistry & Basic Formulating Techniques. Primeaux Associates LLC
Fuger KE, McCoy JJ, Zajacek JG (1975) Process for the manufacture of urethanes. United States patent 3,895,054 A
Tang SLY, Smith RL, Poliakoff M (2005) Principles of green chemistry: PRODUCTIVELY. Green Chem 7(11):761–762. doi:10.1039/b513020b
Kreye O, Mutlu H, Meier MAR (2013) Sustainable routes to polyurethane precursors. Green Chem 15(6):1431. doi:10.1039/c3gc40440d
Guan J, Song Y, Lin Y, Yin X, Zuo M, Zhao Y, Tao X, Zheng Q (2011) Progress in study of non-isocyanate polyurethane. Ind Eng Chem Res 50(11):6517–6527. doi:10.1021/ie101995j
Pan WC, Lin C-H, Dai SA (2014) High-performance segmented polyurea by transesterification of diphenyl carbonates with aliphatic diamines. J Polym Sci A Polym Chem 52(19):2781–2790. doi:10.1002/pola.27302
Hart RE (1996) Water-based, solvent-free or Low VOC, two-component polyurethane coatings. United States Patent 5,508,340
Kubitza W, Gruber H, Probst J (1991) Aqueous coating composition based on specific two-component polyurethanes and to a process for its production. United States Patent 5,075,370
Deepa P, Jayakannan M (2008) Solvent-free and nonisocyanate melt transurethane reaction for aliphatic polyurethanes and mechanistic aspects. J Polym Sci A Polym Chem 46(7):2445–2458. doi:10.1002/pola.22578
Chen H-Y, Pan W-C, Lin C-H, Huang C-Y, Dai SA (2012) Synthesis and trans-ureation of N,N’-diphenyl-4,4′-methylenediphenylene biscarbamate with diamines: a non-isocyanate route (NIR) to polyureas. J Polym Res 19(2):9754–9765. doi:10.1007/s10965-011-9754-8
Lin C-H (2008) Green chemistry synthesis of intermediates for polyurethane-urea. National Chung Hsing University, Taiwan
Qaroush AK, Al-Hamayda AS, Khashman YK, Vagin SI, Troll C, Rieger B (2013) Highly efficient isocyanate-free microwave-assisted synthesis of [6]-oligourea. Catal Sci Technol 3(9):2221. doi:10.1039/c3cy00117b
Ubaghs L (2005) Isocyanate-free synthesis of (Functional) polyureas, polyurethanes, and urethane-containing copolymers. RWTH Aachen University
Murray RJ (1995) Water-based adhesive. United States Patent 5,395,879
Sacks R (2006) Water-based coating. United States Patent 20,060,178,463
Argillier J-f CO, Audibert-hayet A, Zeilinger S (2001) Water-based foaming composition-method for making same. United States Patent 6,172,010
Yeh J-M, Yao C-T, Hsieh C-F, Yang H-C, Wu C-P (2008) Preparation and properties of amino-terminated anionic waterborne-polyurethane–silica hybrid materials through a sol–gel process in the absence of an external catalyst. Eur Polym J 44(9):2777–2783. doi:10.1016/j.eurpolymj.2008.06.040
Xia Y, Larock RC (2011) Preparation and properties of aqueous castor oil‐based polyurethane–silica nanocomposite dispersions through a sol–gel process. Macromol Rapid Commun 32(17):1331–1337
Delebecq E, Pascault JP, Boutevin B, Ganachaud F (2013) On the versatility of urethane/urea bonds: reversibility, blocked isocyanate, and non-isocyanate polyurethane. Chem Rev 113(1):80–118. doi:10.1021/cr300195n
Zastrow A, Witkowski R, Werner R, Kurek G, Rische T, Avtomonov E, Kraus H, Peerlings H (2013) Aqueous polyurethane-polyurea dispersions. Taiwan Patent TW 201206971 A1
Versteegen RM, Kleppinger R, Sijbesma RP, Meijer EW (2006) Properties and morphology of segmented copoly(ether urea)s with uniform hard segments. Macromolecules 39(2):772–783. doi:10.1021/Ma051874e
Orliac O, Rouilly A, Silvestre F, Rigal L (2003) Effects of various plasticizers on the mechanical properties, water resistance and aging of thermo-moulded films made from sunflower proteins. Ind Crop Prod 18(2):91–100. doi:10.1016/s0926-6690(03)00015-3
Anastas P, Eghbali N (2010) Green chemistry: principles and practice. Chem Soc Rev 39(1):301–312. doi:10.1039/b918763b
Acknowledgments
We gratefully acknowledge that this research has been financed by a grant from GRECO of Taichung, Taiwan. We also acknowledge Ms. Karin D. Kelly for editing the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pan, W.C., Liao, K., Lin, CH. et al. Solvent-free processes to polyurea elastomers from diamines and diphenyl carbonate. J Polym Res 22, 114 (2015). https://doi.org/10.1007/s10965-015-0747-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-015-0747-x