Abstract
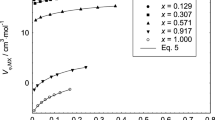
The pressure dependences of the osmotic and activity coefficients of lithium nitrate (LiNO3) in ethanol at 298.15 K up to 40 MPa and 3.0 mol·kg−1 have been estimated from literature volumetric data on LiNO3 solutions in ethanol using the Pitzer ion-interaction or virial coefficient approach. In particular, the volumetric Pitzer ion-interaction parameters (\(\beta_{{{\text{MX}}}}^{{\left( 0 \right){\text{V}}}}\), \(\beta_{{{\text{MX}}}}^{{\left( 1 \right){\text{V}}}}\), and \(C_{{{\text{MX}}}}^{{\text{V}}}\)) have been evaluated from a mathematical fit of literature apparent molar volume data to the appropriate Pitzer equation. These were then expressed as polynomials in pressure, which were used to calculate the change in the osmotic and activity coefficients in going from an initial pressure of p1 to a final pressure of p2. These pressure dependences have subsequently been coupled with the osmotic and activity coefficient values of ethanolic LiNO3 solutions at atmospheric pressure obtained from the literature to yield a comprehensive set of osmotic and activity coefficients for LiNO3 solutions in ethanol at 298.15 K, pressures of 5, 10, 15, 20, 25, 30, 35, and 40 MPa, and molalities to 3.0 mol·kg−1. The approximation as to the pressure dependence of the dielectric constant of ethanol estimated using the isothermal compressibilities has been tested for pressures above 0.1 MPa. The results demonstrate that this approximation becomes poorer with the increase in pressure as far as ethanol is concerned.






Similar content being viewed by others
References
Pitzer, K.S.: Thermodynamics, 3rd edn. McGraw-Hill, Inc., New York (1995)
Pitzer, K.S. (ed.): Activity Coefficients in Electrolyte Solutions, 2nd edn. CRC Press, Boca Raton (1991)
Pramanik, S., Saha, B., Das, B.: Correlation of the volumetric properties of uni-univalent electrolytes in methanol-water mixed solvent media: a pitzer ion-interaction approach. J. Solution Chem. 49, 825–835 (2020)
Pramanik, S., Das, B.: Thermodynamic Properties of aqueous sodium nitrate solutions under superambient conditions. J. Solution Chem. 48, 167–179 (2019)
Das, B.: Pitzer ion interaction parameters of single aqueous electrolytes at 25°c. J. Solution Chem. 33, 33–45 (2003)
Das, B., Pitzer, K.S.: Thermodynamic properties of aqueous potassium fluoride under superambient conditions. J. Solution Chem. 30, 489–496 (2001)
Krumgalz, B.S., Pogorelskii, R., Sokolov, A., Pitzer, K.S.: Volumetric ion-interaction parameters for single-solute aqueous electrolyte solutions at various temperatures. J. Phys. Chem. Ref. Data 29, 1123–1140 (2000)
Das, B., Pitzer, K.S.: Thermodynamic properties of aqueous potassium sulfate under superambient conditions. J. Solution Chem. 28, 283–289 (1999)
Criss, C.M., Millero, F.J.: Modeling heat capacities of high valence-type electrolyte solutions with Pitzer’s equations. J. Solution Chem. 28, 849–864 (1999)
Holmes, H.F., Mesmer, R.E.: Isopiestic molalities for aqueous solutions of the alkali metal hydroxides at elevated temperatures. J. Chem. Thermodyn. 30, 311–326 (1998)
Pitzer, K.S., Das, B.: Thermodynamic properties of Na2SO4 (aq) above 200°C. Geochim. Cosmochim. Acta 62, 915–916 (1998)
Obsil, M., Majer, V., Grolier, J.-P.E., Hefter, G.T.: Volumetric properties of, and ion-pairing in, aqueous solutions of alkali-metal sulfates under superambient conditions. J. Chem. Soc. Farad. Trans. 92, 4445–4451 (1996)
Kim, H.-T., Frederick, W.J., Jr.: Evaluation of Pitzer ion interaction parameters of aqueous electrolytes at 25 °C. 1. Single salt parameters. J. Chem. Eng. Data 33, 177–184 (1988)
Saluja, P.P.S., Pitzer, K.S.P., Phutela, R.C.: High-temperature thermodynamic properties of several 1:1 electrolytes. Can. J. Chem. 64, 1328–1335 (1986)
Pitzer, K.S., Peiper, J.C., Busey, R.H.: Thermodynamic properties of aqueous sodium chloride solutions. J. Phys. Chem. Ref. Data 13, 1–102 (1984)
Rogers, P.S.Z., Pitzer, K.S.: Volumetric properties of aqueous sodium chloride solutions. J. Phys. Chem. Ref. Data 11, 15–81 (1982)
Pitzer, K.S., Mayorga, G.: Thermodynamics of electrolytes. II. Activity and osmotic coefficients for strong electrolytes with one or both ions univalent. J. Phys. Chem. 77, 2300–2308 (1973)
Zafarani-Moatter, M.T., Shekhari, H.: Density and speed of sound of lithium bromide with organic solvents: measurement and correlation. J. Chem. Thermodyn. 39, 1649–1660 (2007)
Das, B.: Thermodynamics of electrolytes in mixed solvent media—Application of the Pitzer ion interaction approach. Can. J. Chem. 83, 2032–2038 (2005)
Yang, J.Z., Xu, W.G.: Medium effect of an organic solvent on the activity coefficients of HCl consistent with Pitzer’s electrolyte solution theory. J. Solution Chem. 34, 71–76 (2005)
Nasirzadeh, K., Papaiconomou, N., Neueder, R., Kunz, W.: Vapor pressures, osmotic and activity coefficients of electrolytes in protic solvents at different temperatures. 1. Lithium bromide in methanol. J. Solution Chem. 33, 227–245 (2004)
Barthel, J., Neuder, R., Wittmann, H.: Osmotic coefficients and activity coefficients of nonaqueous electrolyte solutions. Part 3. Tetraalkylammonium bromides in ethanol and 2-propanol. J. Solution Chem. 28, 1263–1276 (1999)
Barthel, J., Neuder, R., Poepke, H., Wittmann, H.: Osmotic coefficients and activity coefficients of nonaqueous electrolyte solutions. Part 4. Lithium bromide, tetrabutylammonium bromide and tetrabutylammonium perchlorate in acetone. J. Solution Chem. 28, 1277–1287 (1999)
Zafarani-Moattar, M.T., Nasirzade, K.: Osmotic coefficient of methanol + LiCl, + LiBr, and + LiCH3COO at 25 °C. J. Chem. Eng. Data 43, 215–219 (1998)
Israfilov, H., Jannataliyev, R., Safarov, J., Shahverdiyev, A., Hassel, E.: The (p, ρ, T) properties and apparent molar volumes Vϕ of LiNO3 + C2H5OH. Acta Chim. Slov. 56, 95–108 (2009)
Heintz, A.: Recent developments in thermodynamics and thermophysics of non-aqueous mixtures containing ionic liquids. A review. J. Chem. Thermodyn. 37, 525–535 (2005)
Verevkin, S., Safarov, J., Bich, E., Hassel, E., Heintz, A.: Study of vapour pressure of lithium nitrate solutions in ethanol. J. Chem. Thermodyn. 38, 611–616 (2006)
Archer, D.G., Wang, P.: The dielectric constant of water and Debye–Hückel limiting law slopes. J. Phys. Chem. Ref. Data 19, 371–411 (1990)
Srinivasan, K.R., Kay, R.L.: Structural considerations from dielectric measurements on the aliphatic alcohols. J. Solution Chem. 4, 299–310 (1975)
Moriyoshi, T., Ishli, T., Tamai, Y., Tado, M.: Static dielectric constants of water + ethanol and water + 2-methyl-2-propanol mixtures from 0.1 to 300 MPa at 298.15 K. J. Chem. Eng. Data 35, 17–20 (1990)
Sun, T.F., Seldam, C.A.T., Kortbeek, P.J., Trappeniers, N.J., Biswas, S.N.: Acoustic and thermodynamic properties of ethanol from 273.15 to 333.15 K and up to 280 MPa. Phys. Chem. Liq. 18, 107–116 (1988)
Phutela, R.C., Pitzer, K.S.: Densities and apparent molar volumes of aqueous magnesium sulfate and sodium sulfate to 473 K and 100 bar. J. Chem. Eng. Data 31, 320–327 (1988)
Marcus, Y. (ed.): Ion Solvation. Wiley, New York (1985)
Campbell, A.N., Debus, G.H.: Conductances of lithium nitrate solutions in ethyl alcohol and ethyl alcohol–water mixtures at 25.0°C. Can. J. Chem. 34, 1232–1242 (1956)
Robinson, R.A., Stokes, R.H.: Electrolyte Solutions, 2nd edn. Butterworths, London (1959)
Marcus, Y.: The standard partial volumes of ions in solution: part 1. The volumes in single non-aqueous solvents at 298.15 K. J. Mol. Liq. 118, 3–8 (2005)
Acknowledgements
The authors thank the Department of Chemistry, Presidency University, Kolkata, India, to provide the computational facilities.
Author information
Authors and Affiliations
Contributions
Conceptualization: BD; Methodology: SP; Formal analysis and investigation: SP; Writing—original draft preparation: SP; Writing—review and editing: BD; Funding acquisition: BD; Resources: BD; Supervision: BD.
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pramanik, S., Das, B. Osmotic and Activity Coefficients of Lithium Nitrate in Ethanol Under High Pressures. J Solution Chem 51, 1589–1602 (2022). https://doi.org/10.1007/s10953-022-01212-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-022-01212-9