Abstract
In the present study the solution and coordination chemistry of copper(II)–alkyl-N-iminodiacetate systems are studied in aqueous solution by potentiometry, using ion selective copper and pH electrodes, EXAFS (extended X-ray absorption fine structure) and dye probe molecular absorption spectrophotometry. Alkyl-N-iminodiacetates with varying alkyl chain length, methyl (CH3–), n-hexyl (C6H13–), n-dodecyl (C12H25–) and n-octadecyl (C18H37–) were used to tune the amphiphilic properties of the ligands. The polar head groups have both oxygen (hard Lewis base) and nitrogen donor (border-line Lewis base) atoms. This means that metal ions with different bonding characteristics may bind these ligands differently. Furthermore, the chelating properties of the polar head group may be regulated by pH as the acid–base properties of the imine and carboxylic acid groups are different. Copper(II) forms two stable complexes with alkyl-N-iminodiacetates with short alkyl chains, present as monomers in aqueous solution, log10 β1 = 11.10(2), log10 β2 = 19.5(2) for methyl-N-iminodiacetate, and log10 β1 = 12.22(4), log10 β2 = 21.9(2) for n-hexyl-N-iminodiacetate. n-Octadecyl-N-iminodiacetic acid, present as large aggregates in acidic aqueous solution, has short strong hydrogen bonds between carboxylic acid and carboxylate groups in the surface of the aggregates, which hinder complex formation at pH values below 4, obstructs it in the pH region 4–7, while the complex formation behaves as for short-chained alkyl-N-iminodiacetates at pH > 7. The structure around copper in copper(II)–alkyl-N-iminodiacetate complexes in aqueous solution and solid state formed at different pH values and copper(II):alkyl-N-iminodiacetate ratios has been determined by EXAFS. The coordination chemistry of copper(II) shows four strong bonds in the equatorial plane, and two different Cu–O/N bond distances, ca. 0.2 Å apart, in the axial positions of a non-centrosymmetric tetragonally elongated octahedron.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
1 Introduction
For environmental reasons the cleaning of waste-water from industries and purifying plants from heavy metals has caused an increasing interest in efficient metal binding ligands with surface active properties, so called chelating surfactants. These chelating surfactants have several requirements to be fulfilled if they shall function and be able reach the commercial market: (i) being biodegradable and non-toxic for the environment, (ii) the complexation of metal ions must be strong, and at the same time reversible in such a way that both surfactant and metal ion can be separated and recycled, (iii) the synthesis must be readily feasible with environmentally acceptable chemicals and (iv) metal ions must be possible to separate by changing some physico-chemical parameters as e.g. pH, temperature or ionic strength.
It has been reported that copper(II) is more effectively floated and separated with dodecyl-N-iminodiacetic acid than with common surfactants such as lauryl sulfate [1] and that copper(II) complexes with chelating surfactants promote the kinetics at electrodeposition [2]. Carboxylated alkyl polyamines are a group of chemicals used in many technical applications and they fulfil the requirements for chelating surfactants given above. However, these products contain several compounds with varying alkyl chain lengths and different numbers of amine and carboxylate groups. This makes it more or less impossible to perform accurate physical-chemical studies on such products. However, their chemical behavior can be estimated with reasonable accuracy from the properties of well-defined pure model compounds as alkyl-N-iminodiacetates. In this study the amphiphilic properties of the alkyl-N-iminodiacetates are tuned by varying the length of the alkyl chain, –CnH2n+1, abbreviated Cn-IDA, n = 1, 6, 12 and 18. The chelating properties of the polar head group may be regulated by pH as the imine and carboxylic acid groups have significantly different acid–base properties [3]; at dissolution in water the alkyl-N-iminodiacetic acids are converted to zwitter ions where a proton is transferred from one carboxylic acid group to the imine nitrogen as the imine group is a relatively stronger base.
The coordination chemistry of copper(II) is characterised by the Jahn–Teller effect [4], manifested by tetragonally elongated octahedral configuration, 4 short + 2 longer bond distances. It was discovered recently that the Cu–Oax bond distances are different in a series of copper(II) solvates of oxygen donor solvents, including water, as well as in compounds crystallizing in non-centrosymmetric space groups. This is most likely also the case in most of the copper(II) compounds crystallizing in centrosymmetric space groups, but due to random orientation of the axial distances the crystallographic observation is a mean centrosymmetric structure [5]. Copper(II) is a typical border-line electron-pair acceptor forming strong complexes with borderline-soft ligands with nitrogen donor atoms, but it binds also reasonably well to oxygen donor ligands. Previous studies have shown that copper(II) forms two strong complexes with iminodiacetate and alkyl-N-iminodiacetate in aqueous solution, with somewhat stronger complex formation with alkyl-N-iminodiacetic acids than to iminodiacetic acid [6]. All reported complexes have the same principle structure with the nitrogen atom and one of the carboxylate oxygens strongly bound in the equatorial plane while the other carboxylate oxygen is weakly bound at longer distance in the axial positions as expected for a Jahn–Teller distorted complex. Of the atoms bound in the equatorial plane the Cu–O bond distances are slightly shorter than the Cu–N ones due to a smaller atomic radius of oxygen in comparison to the radius of amine nitrogen when binding to copper(II) [7]. A survey of the structures of bis(iminodiacetato/alkyl-N-iminoacetato)cuprate(II) complexes reported in solid state is given in Table S1.
It was observed in a previous study that the alkyl-N-iminodiacetic acids have an extraordinarily short strong hydrogen bond (SSHB) system in the solid state linking carboxylic acid/carboxylate groups from two adjacent molecules into an indefinite sheet structure [3]. These strongly hydrogen bound aggregates prevail to neutral pH in aqueous systems in the case of n-octadecyl-N-iminodiacetic acid, pKa2 ≈ 7, where the van der Waals forces between the closely interdigitated packed alkyl chains cause the back-bone structure to become sufficiently strong and rigid to prevent a break-up of the aggregate in water. In the present study the complex formation between copper(II) and CnH2n+1-IDA ligands, n = 1, 6, 12 and 18, in aqueous solution or suspension has been studied. Of these systems methyl- and n-hexyl-IDA are present as hydrated monomers without significant aggregation, while n-dodecyl- and n-octadecyl-IDA are present as large aggregates, held together with van der Waals forces between the alkyl chains and strong hydrogen bonding between the head groups, Fig. S1. The SSHBs are maintained up to pH = 6–7 in the former, as long as the acid groups are not deprotonated [3]. The influence of this kind of SSHBs on the complex formation of metal ions has not been studied before and may have implications on the view of the complex formation to large molecules (e.g. proteins) able to form aggregates common in biological systems. In order to clarify some of these points the stability constants of the complex formation and the structure around copper(II) in these complexes have been determined. The stability constants have been determined by potentiometric measurements by simultaneous use of ion selective copper and hydrogen (pH) electrodes.
The aim of the present study is to develop the understanding of systems related to copper(II) carboxylated polyamine systems in aqueous solution to increase the separation efficiency by tuning the chemical conditions. In order to perform accurate studies, alkyl-N-iminodiacetates with different length of the alkyl chain have been chosen as they can be prepared pure and accurate physico-chemical measurements can be performed. The stability constants, the acid–base properties and the structures of the copper(II) alkyl-N-iminodiacetate systems have been determined in this study. The structural studies of copper(II)–Cn-IDA complexes have been performed by EXAFS (extended X-ray absorption fine structure) on both solids and aqueous solutions with varying pH, length of alkyl chain and stoichiometry. With this information it is possible to more accurately predict the properties of commercial carboxylated polyamine compounds to separate copper(II) compounds from e.g. waste waters.
2 Experimental Section
2.1 Chemicals
Iminodiacetic acid, HN(CH2COOH)2 (Aldrich, 99%), methyl-N-iminodiacetic acid, CH3N(CH2COOH)2 (Aldrich, 99%), copper(II) perchlorate hexahydrate, [Cu(H2O)6](ClO4)2 (reagent, GFS Chemicals, ≥ 99%), copper(II) nitrate hexahydrate, [Cu(H2O)6](NO3)2 (Merck, analytical grade, ≥ 99%), sodium perchlorate monohydrate, NaClO4·H2O (Merck, analytical grade, ≥ 99.5%), sodium hydroxide solution, NaOH, (Merck, Titrisol, ≥ 99.9%), and ethylenediaminetetraacetic acid, disodium salt, (Merck, ≥ 99.9%) were used as purchased. n-Hexyl-N-iminodiacetic acid, n-C6H13N(CH2COOH)2, n-dodecyl-N-iminodiacetic acid, n-C12H25N(CH2COOH)2, and n-octadecyl-N-iminodiacetic acid, n-C18H37N(CH2COOH)2, were synthesised in very high purity as described elsewhere [3]. The water used in this study has been deionized and Milli Q filtered with 18.2 MΩ·cm resistance.
2.2 Methods
2.2.1 Potentiometry
The free copper(II) concentration was monitored by an ion selective Orion model 9629 Ion Plus series cupric electrode with an Orion 720A potentiometer. Reference filling solution was 0.12 mol·dm−3 NaClO4. The standard solutions were 1.0 × 10−6, 1.0 × 10−5, 1.0 × 10−4, 1.0 × 10−3, 1.0 × 10−2 and 1.0 × 10−1 mol·dm−3 copper(II) perchlorate in the presence of 0.100 mol·dm−3 NaClO4 as supporting electrolyte. Calibration of the cupric selective electrode was performed both before and after the titration experiments. The titrator solution was added manually from a digital burette Metrohm 665 Dosimat. The sample chamber was covered with aluminium foil to ensure constant and almost dark conditions, as the sensing element may show an offset if the light conditions change markedly. pH was measured using an Orion 9102AP gel filled glass pH electrode. Calibrations were performed with Orion standards at pH = 4.01, 7.00 and 10.01. The measurements were performed in jacketed glass vessels (Methrohm) through which thermostated water (298.0 ± 0.1 K) continuously flowed. The measurements and calibrations were performed in nitrogen atmosphere (very gentle flow) and gentle magnetic stirring. The deionized water used was boiled for some minutes before the sample preparation to release dissolved gas as the electrode is sensitive to gas bubbles on the sensing element. The acidity constants were calculated from pH titration data by means of the program MAINPH [8], and the stability constants were evaluated from metal titration data with the program EMFALL [8].
2.2.2 Extended X-ray Absorption Fine Structure (EXAFS)
The Cu K-edge EXAFS measurements were performed at the Stanford Synchrotron Radiation Lightsource (SSRL), USA. SSRL operated at 3.0 GeV and a maximum current of 100 mA. The EXAFS station at the wiggler beam line 4-1, old station, was equipped with a Si[220] double crystal monochromator. The solids and the aqueous copper(II) solutions were simultaneously measured in both fluorescence and transmission mode. Internal calibration was made with a copper metal foil assigning the first inflection point on the edge to 8980.3 eV [9], and higher order harmonics were discarded by detuning the second monochromator crystal to 50% of maximum intensity at the end of the scans. The data treatment, using standard procedures for pre-edge subtraction and spline removal, and model fitting of the EXAFS data has been carried out by use of the EXAFSPAK program package [10]. The resulting EXAFS functions have been curve-fitted by calculated model functions using ab initio calculated EXAFS phase and amplitude parameters from FEFF7 (ver. 7.02) [11, 12].
EXAFS measurements were performed on solids precipitated at mixing of 0.5 mol·dm−3 copper(II) perchlorate solutions with concentrated alkyl-N-iminodiacetic acid solutions adjusted to predetermined pH values with a 5.0 mol·dm−3 sodium hydroxide solution. EXAFS measurements were also performed on two 0.5 mol·dm−3 aqueous solutions. The EXAFS data on the aqueous solutions were collected at a higher concentration than the potentiometric data in order to obtain as good data quality as possible. The chosen concentration in the EXAFS studies did insure that the amount of water was sufficient to have free water as solvent, and good EXAFS data quality. As long as the concentration of free water is high it is assumed that the speciation is similar to the one obtained in the potentiometric studies.
2.2.3 Determination of Stability Constants of Alkyl-N-iminodiacetic acid (Cn-IDA, n = 1,6) Complexes
Titrations of 50 cm−3 10 mmol·dm−3 copper(II) perchlorate in 0.100 mol·dm−3 sodium perchlorate using 0.100 mol·dm−3 methyl- or n-hexyl-N-iminodiacetic acid as titrator were performed. The pH of the titrator solution was adjusted by adding one equivalent of sodium hydroxide to the isoelectric form of the ligand to a final pH of 7–8.
2.2.4 Determination of Stoichiometries of Copper(II)-n-octadecyl-N-iminodiacetic Acid Complexes
Titrations of 100 cm−3 0.5 mmol·dm−3 n-octadecyl-N-iminodiacetic acid in 100 mmol·dm−3 sodium perchlorate suspension, obtained after ultrasonication for one hour, and using 10 mmol·dm−3 copper(II) perchlorate in 80 mmol·dm−3 sodium perchlorate as titrant, were performed. The starting pH of the n-octadecyl-N-iminodiacetic acid suspension was adjusted to 4.00, 6.40 and 9.50 by addition of an appropriate amount of 0.100 mol·dm−3 sodium hydroxide solution.
3 Results
3.1 Stability of Copper(II)–Alkyl-N-iminodiacetic Acid (Cn-IDA, n=1,6) Complexes
Determination the stability constants of the copper(II) methyl- and n-hexyl-N-iminodiacetate systems in aqueous solution has been made by potentiometry. The free copper(II) and hydrogen concentrations show a coherent behavior versus added ligand in both systems. pH decreases until one equivalent of ligand is added versus copper(II), giving a pH value of ca. 2.5; thereafter, the pH rises slightly again. Small distinct differences in the resulting pH curves between copper(II)-methyl- and -n-hexyl-N-iminodiacetic acid titrations are seen. The stability constants obtained in this study and selected values reported in the literature are summarized in Table 1. The stability constants of copper(II)–alkyl-N-diacetate systems increases with increasing length of alkyl chain using the same ionic medium, Table 1. A possible reason is that the hydration of the formed CuL and CuL2 complexes decreases with increasing alkyl chain length. The water molecules around longer alkyl chains have to rearrange, which require energy, and the forces between the alkyl chains and the water molecules are very weak. On the other hand, the protonated imino nitrogen in iminodiacetate can form hydrogen bonds with surrounding water molecules.
3.2 Acid–Base Properties of Copper(II) Alkyl-N-iminodiacetate Complexes
3.2.1 pH Titrations
The acid-base titration curves of copper(II)–alkyl-N-iminodiacetic acid complexes at the stoichiometry 1:2 present three principal different behaviors of the four ligands, iminodiacetic acid and methyl-, n-hexyl- and n-dodecyl-N-iminodiacetic acid, Fig. 1. The copper(II)–methyl- and n-hexyl-N-iminodiacetate systems display common features, however, with a significant difference in the buffer region between the 3rd and 4th equivalence points, indicating that one of the alkyl-N-iminodiacetic acid groups bound to copper(II) remains protonated below pH = 5–6. The buffer region is ca. one pH unit lower for the copper(II)–methyl-N-iminodiacetic acid system than for the copper(II)–n-hexyl- and n-dodecyl-N-iminodiacetic ones. The copper(II)–n-dodecyl-N-iminodiacetic acid system displays additional buffer capacity in the region between the 2nd and 3rd equivalence points. This buffer region is also observed in the titration curve of the pure n-dodecyl–N-iminodiacetic acid system as shown in Fig. 8 in [3], strongly indicating that this buffer region is caused by the SSHBs as it is also observed for the n-octadecyl-N-iminodiacetic acid system. This shows that the SSHB in aggregates of n-dodecyl- and n-octadecyl-N-iminodiacetic acid prevent complex formation with copper below pH values of 6 and 4, respectively. These observations show that the following equilibria with calculated acidity constant (pKa) exists in the region between the 3rd and 4th equivalence points:
Potentiometric titration of copper–alkyl-N-iminodiacetic acid systems at stoichiometry 1:2, a 10 mmol·dm−3 Cu(ClO4)2/20 mmol·dm−3 iminodiacetic acid in 0.100 mol·dm−3 NaClO4, (experimental data points-filled black triangles, and modelled function-line), b 10 mmol·dm−3 Cu(ClO4)2/20 mmol·dm−3 methyl-N-iminodiacetic acid in 0.100 mol·dm−3 NaClO4, (experimental data points-filled black diamonds, modelled function-line), c 10 mmol·dm−3 Cu(NO3)2/20 mmol·dm−3 n-hexyl-N-iminodiacetic acid in 0.100 mol·dm−3 NaClO4, (experimental data points-filled black squares, modelled function-line) and d 10 mmol·dm−3 Cu(NO3)2/20 mmol·dm−3 n-dodecyl-N-iminodiacetic acid in 0.100 mol·dm−3 NaClO4 (experimental data points-filled black circles, modelled function-line)
pKa2 = 5.35, 6.37, 6.36, for A = CH3-, C6H13- and C12H25-IDA, respectively.
When the pH is raised above the 4th equivalence point (pH > 10), there is no buffer capacity left in any of the systems. This shows that copper(II)–n-alkyl-N-iminodiacetate complexes with the stoichiometry 1:2 persist at high pH values, which is supported by the observation that no precipitation of hydrolysis products is observed at pH < 13. In the case of the copper(II)–C12-IDA system a precipitation takes place at higher pH, but this is a blue voluminous precipitate in contrast to the light-blue fine-grained copper(II) hydroxide. It is noticeable that the copper(II)–n-hexyl- and –n-dodecyl-N-iminodiacetic acid systems have very similar values of pKa despite the different behavior at lower pH in the region between the 2nd and 3rd equivalence points, due to presence of short strong hydrogen bonds in the fraction of n-dodecyl-N-iminodiacetic acid present as aggregates.
The imino hydrogen of the first alkyl-N-iminodiacetate bound to copper(II) is very acidic, and copper(II) is not out-competed when pH is above 1.5, Fig. S2. The acidity of the imino hydrogen decreases of the second alkyl-N-iminodiacetic acid ligand bound to copper(II), but still a substantial shift of the basicity remains in comparison with free alkyl-N-iminodiacetic acid, ca. 4–5 orders of magnitude (see above). The complex distribution functions for the copper(II)–methyl- and –n-hexyl-N-iminodiacetate systems in water, as function of ligand and copper(II) species, are shown in Fig. S2.
The copper(II)–iminodiacetate system shows a somewhat different behavior in the buffer region, between the 3rd and 4th equivalence points, in comparison to the copper(II)–alkyl-N-iminodiacetic acid complexes in the region, Fig. 1. According to literature data, the stepwise stability constant of the complex formation of copper(II) iminodiacetate hydroxide is somewhat higher than for the formation of the di(iminodiacetato)cuprate(II) complex, the log10 K values are 6.26 and 6.05, respectively [17, 21, 27]. A similar observation has also been made for a water–alcohol mixture [28]. It is therefore more likely that a hydroxide ion binds to copper(II) than a second iminodiacetate at neutral pH. This means that similar amounts of the complexes \({\text{Cu}}\left( {{\text{IDA}}} \right)_{2}^{2 - }\) and Cu(IDA)OH− are present at neutral pH and that the buffer capacity will be split into two parts, the formation of a copper(II) iminodiacetate hydroxide complex, and deprotonation of free ammoniumdiacetate (the form of free ligand present in the pH region 3–8), pKa3 ≈ 9.2. The reported stability constants should give a distribution of 60 % copper(II)–iminodiacetato-hydroxido complex, and 40 % bis(iminodiacetato)cuprate(II) complex giving 30 % of the total ligand concentration as free ligand, when total ligand concentration is twice the total copper(II) concentration at neutral pH. This pattern is indeed very nicely seen in the titration of iminodiacetic acid with sodium hydroxide, and the pKa value of iminodiacetate bound to copper(II) can be calculated to 5.75(5), and the acidic constants of iminodiacetic acid are in full agreement with literature values, ca. 2.55 and 9.3 for pKa1 and pKa2, respectively, Fig. 1. To secure the presence of [Cu(IDA)2]2− and [Cu(Cn-IDA)2]2− an excess of more than a factor of two of IDA or Cn-IDA is required.
The copper(II)–n-octadecyl-N-iminodiacetic acid system was studied at initial pH values of 4.0, 6.3 and 9.5, in order to study the effect of SSHBs on complex formation. At pH = 4.0 no complex formation was observed at all, while copper(II) is strongly bound to the aggregates at pH = 9.5, Fig. 2. Titrations starting at pH = 6.3 show significant complex formation at the start, which causes a decrease in pH. After some additions, when the pH value has reached pH ca. 5.0, it takes very long time, hours, to reach stable copper and pH potentials. This shows clearly that the SSHBs obstruct the complex formation at pH values lower than 4–5, and that the steric restrictions seem to be very important. When approximately 50 % of the coordination sites are coordinated by a copper(II) ion, the rate of the complex formation increases more or less independently of pH. This indicates that the coordinating copper(II) ions break up the rigid structure of the SSHB linked alkyl-N-diiminodiacetic acid structure into a structure where the hydration and complex formation processes have better conditions. These results show clearly that the presence of SSHBs in the ligand can significantly hamper the ability of the ligand to participate in protonation and complex formation reactions, and in fact hinder the complex formation at low pH values.
The acid–base titration curves of copper(II) iminodiacetic and n-hexyl-N-iminodiacetic acid complexes at the stoichiometry 1:1 have been performed, Fig. S3. The first equivalence point is defined as one equivalent of base added per copper(II). The titration curve of the copper(II)–n-hexyl-N-iminodiacetic acid system show a large pH rise when two equivalents of hydroxide (vs. ligand) are added, which is expected, as the form of the added ligand is a diprotonic acid. However, a buffer region is obtained between the 2nd and 3rd equivalence points, and a pale blue precipitate, probably a mixed copper(II) n-hexyl-N-iminodiacetate hydroxide compound, is observed.
3.2.2 Determination of Copper(II)–Ligand Stoichiometry for Ligands Present as Aggregates in Solution
A method to determine the concentration of ligands forming strong complexes with copper(II) in the low μmol·dm−3 range, dye probe molecular absorption spectrophotometry, was developed (see ESI section (ESI†)). This method allows estimation of the stoichiometry of the copper(II)–analyte complex from the slope in the calibration curve using the wavelength 566 nm. The ligands n-hexyl-, n-dodecyl- and n-octadecyl-N-iminodiacetic acid have been investigated in this study, Fig. S4. The results indicate two principal behaviors of the copper(II)–alkyl-N-iminodiacetic acid systems. The n-hexyl- and n-dodecyl-N-iminodiacetic acids have similar slopes in the calibration curves, indicating formation of a copper(II)–n-hexyl-iminodiacetic acid complex with the stoichiometry 1:1, which is expected from the concentrations and the pH value used, ~ 6.8, and the stability constants were determined potentiometrically, Table 1. However, there is a small finite difference between the ligands, indicating formation of a small amount of a second complex with n-dodecyl-N-iminodiacetate, as expected from the relatively more stable second complex, Table 1. The calibration curve of n-octadecyl-N-iminodiacetate presents a principal different behavior, both in comparison to other ligands and over time. The measurements were repeated at four different times, at 1, 6 and 24 h and 6 days after preparation of the solutions. In the low concentration range the intensity is almost independent of the concentration of n-octadecyl-N-iminodiacetate. In the concentration range 0–24 μmol·dm−3 the slope indicates a formal stoichiometry of 1:5. This anomalously high value indicates that only ca. each fifth available site in the aggregate surface of n-octadecyl-N-iminodiacetic acid is occupied by a copper(II) ion. The presence of SSHBs in the aggregates of n-octadecyl-N-iminodiacetate below pH = 6–7 shows that copper(II) ions in the studied concentration range, 0–46 µmol·dm−3, and pH ≈ 6.8, have low possibility to enter the coordination sites in the aggregate surface even after 6 days.
At concentrations exceeding 46 µmol·dm−3, a large drop in absorbance is observed, enhanced dramatically after 6 days. At concentrations above 54 µmol·dm−3, another copper(II) independent region is seen at shorter times, even a weak increase in absorbance is observed at around a twofold excess of n-octadecyl-N-iminodiacetic acid versus copper(II). However, the fundamental shift in the calibration curves is at concentrations exceeding 46 µmol·dm−3 n-octadecyl-N-iminodiacetic acid (vs. 56 µmol·dm−3 copper(II)-ECR) at extended times. The slope of the curve indicates that a stoichiometry of almost 1:1 is obtained, indicating that almost all coordination sites in the aggregate surface of n-octadecyl-N-iminodiacetic acid bind copper(II) ions.
In a solution with n-octadecyl-N-iminodiacetic acid:copper(II) ratios larger than 1.0, Cu(C18-IDA)2 complexes (1:2) are slowly formed with time, 6 days, Fig. S4. This shows that copper(II) ions can break up the SSHBs in the C18-IDA aggregates also in neutral solution and at low total concentrations, but it takes several days.
3.2.3 Structure Determination using Extended X-ray Absorption Fine Structure (EXAFS)
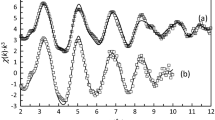
EXAFS has been used to study the structure around copper(II) in the copper(II)–alkyl-N-iminodiacetic acid complexes with varying length of the hydrocarbon chain (methyl, n-hexyl, n-dodecyl and n-octadecyl), pH and stoichiometry. The features of the EXAFS modulations of the studied samples are surprisingly similar, in spite of different compositions and varying lengths of the alkyl chain (Fig. 3). Copper(II) binds strongly to four oxygens/nitrogens, certainly two of each, in the equatorial plane at ca. 1.97 Å, and additionally two oxygens from the acetate groups. The refinements of the experimental data gave a better fit with two different bond distances in the axial positions as is also found in the hydrated copper(II) ion in aqueous solution [5]. The difference in the Cu–Oax bond distances is in the order of 0.2 Å, Tables 2 and S4 (ESI†). In addition to the single scattering to methylene, \({\text{C}}_{{{\text{H}}_{2} }}\), and carboxylate, C=O, carbons in the second scattering shell, ca. 2.8 Å, in the alkyl-N-iminodiacetate ligand, there is complex multiple scattering within the atoms in the alkyl-N-iminodiacetate ligands. These include Cu–O–C=O–Cu and \({\text{Cu}}{-}{\text{N}}{-}{\text{C}}_{{{\text{H}}_{2} }}{-}{\text{Cu}}\) at ca. 3.2 Å (approximate mean value), Cu–C=O–N–Cu, \({\text{Cu}}{-}{\text{O}}{-}{\text{C}}_{{{\text{H}}_{2} }}{-}{\text{Cu}}\) and \({\text{Cu}}{-}{\text{C}}_{{ = {\text{O}}}}{-}{\text{C}}_{{{\text{H}}_{2} }}{-}{\text{Cu}}\) at ca. 3.65 Å (approximate mean value). As there are two different Cu–Oax bond distances, there will be a fairly large number of different distances to the carbons in the second scattering shell and in the 3-leg scattering paths. The distances to carbons in the second scattering shell and the 3-leg scattering paths cannot be separated from each other and have therefore been refined to mean values; also, it is not possible to distinguish between carbon, nitrogen and oxygen atoms as back-scatterers. The light blue solids precipitating from aqueous solutions with equal concentrations of copper(II) and alkyl-N-iminodiacetic acid at pH = 2 seem to form \(\left( {{-}{\text{Cu}}\left( {{\text{OH}}_{2} } \right)_{m}{-}{\text{O}}{-}{\text{C}}\left( {\text{O}} \right){-}{\text{CH}}_{2}{-}{\text{N}}\left( {{\text{RH}}} \right){-}{\text{CH}}_{2}{-}{\text{C}}\left( {\text{O}} \right){-}{\text{O}}} \right)_{n}^{ + }\), m + n = 6, chains where the imine nitrogen is protonated, and thereby not binding to copper(II); supported by their light blue color. The aqueous solutions with a fivefold excess of ligand, and solids precipitated from such solutions, all have an intense blue color indicating that at least one, but most like likely two, nitrogen atoms bind to copper. The bis(alkyl-N-iminodiacetato)copper(II) complexes are singly protonated below pH ca. 5.5 (see above), which means that the nitrogen on one of the ligands is protonated, and thereby not binding to copper(II). Above ca. pH = 5.5 the nitrogen atoms in both ligands are deprotonated and most likely bound to copper. However, the geometry and bond distances around the copper(II) change only marginally at deprotonation and simultaneous coordination of the nitrogen to copper in the second ligand.
The fit of EXAFS data of a, solid copper(II) di(methyl-N-iminodiacetate) complex precipitated from an aqueous solution with a fivefold excess of ligand and pH = 5 (offset: 20), b an aqueous solution of a copper(II) methyl-N-iminodiacetate complex with a fivefold excess of ligand and pH = 5 (offset: 18), c solid copper(II) n-hexyl-N-iminodiacetate compound precipitated from an aqueous solution with equal concentrations of copper(II) and ligand at pH = 2 (offset: 16), d an aqueous solution with equal concentrations copper(II) and n-hexyl-N-iminodiacetate at pH = 2 (offset: 14), e solid copper(II) di(n-hexyl-N-iminodiacetate) complex precipitated from an aqueous solution with a fivefold excess of ligand at pH = 7 (offset: 12), f a gel of copper(II) di(n-hexyl-N-iminodiacetate) complex at pH = 2 (offset: 10), g solid copper(II) di(n-hexyl-N-iminodiacetate) complex precipitated from an aqueous solution with a fivefold excess of ligand at pH = 13 (offset: 8), h solid copper(II) n-dodecyl-N-iminodiacetate complex precipitated from an aqueous solution with the stoichiometry 1:1 and pH = 2 (offset: 6), i solid copper(II) di(n-dodecyl-N-iminodiacetate) complex precipitated from an aqueous solution with a fivefold excess of ligand at pH = 2 (offset: 4), j solid copper(II) di(n-dodecyl-N-iminodiacetate complex precipitated from an aqueous solution with a fivefold excess of ligand and pH = 5 (offset: 2), and k solid copper(II) di(n-octadecyl-N-iminodiacetate) complex precipitated from an aqueous suspension with a fivefold excess of ligand at pH = 6 (no offset), experimental data—thin solid line with data points as open diamonds and calculated model function using the parameters given in Table 2 as thick solid line
These changes in the structure are not large enough to be detectable with the EXAFS technique. The results show very clearly that the length of the alkyl chain does not directly influence the structure around the copper(II) ion.
The most important refined structure parameters from the EXAFS data are summarized in Table 2, and full details are given in Table S2, the fits of the raw EXAFS data are shown in Fig. 3, and of the Fourier transforms in Fig. S4. The structure parameters given in Tables 2 and S2, and the given estimated errors are the statistical ones obtained in the refinement procedure. The estimated total errors, including statistical, systematic and in the Eo value (for which k = 0 Å−1), are given in the text. The total errors of well-defined distances are in the range ± 0.01–0.02 Å.
4 Discussion
The alkyl-N-iminodiacetic acids form extraordinary short strong hydrogen bonds (SSHBs) between an acetic acid/acetate-pair in two adjacent neutral zwitterionic alkyl-N-iminodiacetic acids (alkyl-N-ammoniumacetateacetic acid) forming indefinite sheets in the solid state [3]. These SSHBs break up in aqueous solution for alkyl-N-iminodiacetic acids with a short alkyl chain, and the acidic constants are in the range of those expected for this type of compounds [3]. However, for the compounds with long alkyl chains the forces between the interdigitating alkyl chains are sufficiently strong to maintain the sheet structure in aqueous suspensions and this means that the SSHBs can persist in aqueous systems up to pH ≈ 7 for n-octadecyl-N-iminodiacetic acid and to pH ≈ 4 for n-dodecyl-N-iminodiacetic acid with the support of the rigid and stable structure of the long alkyl chains [3]. This causes the acidity of the alkyl-N-iminodiacetic acids, present as large sheets in aqueous suspensions, to be much weaker than those present as monomers with a factor of ca. 104–105 [3]. This study shows also that the complex formation is strongly influenced by the presence of SSHBs in the ligand system. The complex formation between copper(II) and monomeric alkyl-N-iminodiacetate is strong when the ligands are present as monomers, and complex formation takes place readily also in weakly acidic solution. On the other hand, when the alkyl-N-iminodiacetic acid is present as aggregates the hydrogen bonding in the surface of the aggregates will strongly influence the complex formation properties. At low pH values where the SSHB is complete, below pH ≈ 4, the hydrogen bonds seem to induce a configuration that does not allow a metal ion to attack and form a complex. In the pH region 4–7 the kinetics are very slow, indicating that a partial deprotonation of the carboxylic acid groups in the aggregate surface changes the configuration of the surface and the possibility for an attack increases. Complex formation causes a decrease in pH, due to the proton release in the complex formation reaction, which causes the reaction/reaction rate to slow down after coordination of metal ions. If less than ca. 50% of the binding sites bind copper(II) ion then the complex formation reaction may stop if the pH value is close to 4 or lower, and the configuration of the surface is similar to that of the fully protonated surface. On the other hand, if more than 50% of the sites bind a copper(II) ion then the configuration is broken up, and the complex formation proceeds slowly even at pH values close to 4. At pH values above 7 the surfaces are deprotonated and fully open for attack from metal ions, and the complex formation proceeds, in principle, in the same way as for the monomers.
Comparisons of the kinetics of the substitution reaction between the hydrated copper(II) ion and the ethylenediaminetetraacetatonickelate(II) ion (EDTA–Ni(II)) [28] and the cyclohexylenediaminetetraacetatonickelate(II) ion (CDTA–Ni(II)) [29] at low pH, have been reported. In contrast to the dominant role of the copper(II) ion attack in the reaction with (EDTA–Ni(II)), there is no copper(II) ion attack of CDTA–Ni(II) below pH = 4. The results were explained in terms of steric hindered rotation and blocking by the backbone structure in cyclohexylenediaminetetraacetate. The proposed mechanism is that the nickel–nitrogen bond dissociation is the rate-determining step. The proposed reaction mechanism for complexation of polyamines to hydroxycuprate(II) in basic media is an initial interaction of a nitrogen donor atom at an axial position, followed by Jahn–Teller inversion to the basal plane [31,32,32]. The rate-determining step in this system appears to be the inversion step. In the present study, it is observed in the titration starting at pH = 9.5, a plateau around pH = 5, which is kinetically controlled, Fig. 2. The amount of bound copper(II) is independent of free copper(II) concentration. The same independence of copper(II) concentration is also observed in the titration with onset pH = 4.0. However, these two titrations with different starting pH represent two principal different systems, the absence and presence of hydrogen bonded aggregates. When starting at pH = 9.5 (~ pKa3), it is expected that only weak van der Waals forces hold the aggregates together, and the acetate and imino groups are available to coordinate metal ions. At such conditions copper(II) will form strong complexes, and no sterical restrictions seems to exist in the system at these high pH values. At pH = 5 copper(II) complexes with the stoichiometry 1:2 (each second coordination site binds copper(II)) are formed, and one of the coordinating n-octadecyl-N-iminodiacetic acid ligands are protonated at the imino nitrogen. A region with significant buffer capacity, pH ≈ 5, is observed in the copper(II)–n-octadecyl-N-iminoacetate system. The fact that this system has buffer capacity at pH = 5 shows that the imino nitrogen is exposed to the surrounding solution and open to copper attack, and that the remaining proton on the nitrogen is affected by the presence of copper ions. The slow kinetics observed when copper(II) binds to the aggregate and at least half of the positions already are occupied suggest that the initially formed copper complexes are fairly stable. The proposed mechanism for the substitution reaction between the hydrated copper(II) ion and the ethylenediaminetetraacetatonickelate(II) ion shows that the nickel–nitrogen bond dissociation is the rate-determining step. In the present study, a copper(II) ion, forming 1:2 complexes at pH = 5, seems to block another hydrated copper(II) ion to enter and thereby hinder the formation of a 1:1 complex. The slow kinetics may be caused by steric restrictions of the backbone structure of the long-tailed hydrocarbon. If intermolecular hydrogen bonded aggregates are already present, complex formation is hindered below pH ≈ 4, and obstructed in the pH range 4–7.
The coordination geometry around copper(II) follows a strict pattern with one nitrogen and oxygen atom from each iminodiacetate ligand binding strongly in the equatorial plane, and the third carboxylate oxygen atom weakly bond in the axial positions with different Cu–O bond distances of Jahn–Teller distorted octahedral configuration. At low pH, pH<5, the imino nitrogen becomes protonated and the Cu–N bond breaks. The coordination of iminodiacetate to copper(II) lowers the acidic constant from ca. 10−9 to 10−5 mol·dm−3 of the imino nitrogen. Because of this, instead of two stable 5-membered chelate rings, a significantly less stable 8-coordinated chelate ring is formed, Scheme 1. The alkyl back-bone and its organization seems not to affect the coordination around copper(II).
References
Allen, W.D., Jones, M.M., Mitchell, W.C., Wilson, J.D.: Adsorbing colloid flotation of Cu(II) with a chelating surfactant. Sep. Sci. Technol. 14, 769–776 (1979)
Eivazihollagh, A., Bäckström, J., Norgren, M., Edlund, H.: Electrochemical recovery of copper complexed by DTPA and C12-DTPA from aqueous solution using a membrane. Cell. J. Chem. Technol. Biotechnol. 93, 1421–1431 (2018)
Häggman, L., Lindblad, C., Oskarsson, H., Ullström, A.-S., Persson, I.: The influence of short strong hydrogen bonding on the structure and the physicochemical properties of alkyl-N-iminodiacetic acids in solid state and aqueous systems. J. Am. Chem. Soc. 125, 3631–3641 (2003)
Bersuker, I.B.: Electronic Structure and Properties of Transition Metal Compounds. Wiley-Interscience, New York (1996).. (Chaps. 7.3 and 9.4)
Persson, I., Lundberg, D., Bajnoczi, É.G., Klementiev, K., Just, J., Sigfridsson Clauss, K.G.V.: EXAFS study on the coordination chemistry of the solvated copper(II) Ion in a series of oxygen donor solvents. Inorg. Chem. 59, 9538–9550 (2020)
IUPAC stability constants database, Academic Software, Sourby Old Farm, Timble, Otley, Yorks, LS21 2PW, UK (2016). http://www.acadsoft.co.uk
Persson, I., Penner-Hahn, J.E., Hodgson, K.O.: An EXAFS spectroscopic study of solvates of copper(I) and copper(II) in acetonitrile, dimethyl sulfoxide, pyridine and tetrahydrothiophene solution and a large angle X-ray scattering study of the copper(II) acetonitrile solvate in solution. Inorg. Chem. 32, 2497–2501 (1993)
Sandell, A.: Physical Chemistry 1, Chemical Center, Lund University, Sweden. Available from the corresponding author of this paper. Personal communication
Thompson, A., Attwood, D., Gullikson, E., Howells, M., Kim, K.-J., Kirz, J., Kortright, J., Lindau, I., Liu, Y., Pianetta, P., Robinson, A., Scofield, J., Underwood, J., Williams, G., Winick, H.: X-ray Data Booklet, 3rd edn. Lawrence Berkley National Laboratory, Berkeley (2009)
George, G.N., Pickering, I.J.: EXAFSPAK—A Suite of Computer Programs for EXAFS Analysis, SSRL. Stanford University, Stanford (1993)
Zabinsky, S.I., Rehr, J.J., Ankudinov, A.L., Albers, R.C., Eller, M.J.: Multiple scattering calculations of X-ray absorption spectra. Phys. Rev. B 52, 2995–3009 (1995)
Ankudinov, A. L., Rehr, J. J.: Relativistic calculations of spin-dependent X-ray absorption spectra. Phys. Rev. B 56, R1712–R1716 (1997). The FEFF program available from http://feff.phys.washington.edu/feff
Gaizer, F., Lazar, J., Kiss, J., Poczik, E.: Protonation and complex formation equilibria of n-(phenylcarbamoylmethyl)iminodiacetic acid derivatives. 1. The complexes of HIDA and diethylcarbamoyl-MIDA. Polyhedron 11, 257–264 (1992)
Nair, M.S., Arasu, P.T., Margret, R.J., Sudha, C., Pillai, M.S.: Studies on copper(II) and zinc(II) mixed-ligand complexes involving some potentially tridentate ligands. Indian J. Chem. Sect. A 31, 865–869 (1992)
Rajathiyumoni, P., Nair, M.S., Arusu, P.T., Thillai, P.: Heterobinuclear complex formation by iminodiacetic acid and DL-2,3-diaminopropionic acid with some transition metal ions. Indian J. Chem. Sect. A 31, 760–763 (1992)
Ullah, M.R., Bhattacharya, P.K.: Studies of some ternary complexes of copper(II) Involving tridentate and monodentate ligands. Indian J. Chem. Sect. A 30, 976–978 (1991)
Hulanicki, A., Krawczynski, T., Krawczyk, V., Lewenstam, A.: Effect of some chelating ligands on the potential response of the chalcocite copper ion selective electrode. Anal. Chim. Acta 158, 343–355 (1984)
Stella, R., Valentin, M.T.G.: A study of copper and cadmium iminodiacetate complexes by ion selective electrodes and application to cadmium monitoring. Anal. Chim. Acta 152, 191–202 (1983)
Ramanujam, V.V., Selvarajan, V.M.: Equilibrium studies of formation of mixed-ligand chelates of copper(II), nickel(II), zinc(II) and cadmium(II) in solution. J. Indian Chem. Soc. Sect. A 58, 125–128 (1981)
Bonomo, R.P., Cali, R., Riggi, F., Rizzarelli, E., Sammartano, S., Siracusa, G.: Thermodynamic and spectroscopic properties of mixed complexes in aqueous-solution—copper(II) complexes of 2,2′-bipyridyl and iminodiacetic or pyridine-2,6-dicarboxylic acid. Inorg. Chem. 18, 3417–3422 (1979)
Anderegg, G.: Komplexone 36. Reaktionsenthalpie und -entropie bei der Bildung der Metallkomplexe der Hoheren edta Homologen. Helv. Chim. Acta 47, 1801–1814 (1964)
Chaberek, S., Martell, A.E.: Stability of metal chelates. 1. Iminodiacetic and Iminodipropionic acids. J. Am. Chem. Soc. 74, 5052–5056 (1952)
Verdier, E., Piro, J.: Determination of formation constants of metal ion complexes by polarography. Ann. Chim. (France) 4, 213–218 (1969)
Schwarzenbach, G., Anderegg, G., Schneider, W., Senn, H.: Komplexone. 26. Uber die Koordinationstendenz von n-Substituierten Iminodiessigsauren. Helv. Chim. Acta 38, 1147–1170 (1955)
Israeli, M., Pettit, L.: Coordination of silver(I) to olefinic bonds—complex-formation between cobalt(II), nickel(II), copper(II), zinc(II), cadmium(II), and silver(I), and some unsaturated derivatives of acetic and iminodiacetic acids. J. Chem. Soc. Dalton Trans. (1975). https://doi.org/10.1039/DT9750000414
Souchay, P., Israily, N., Gouzerh, P.: Preparation et etude en solution de nouveaux amino diacides complexants. Bull. Soc. Chim. Fr. 12, 3917–3923 (1966)
Leach, B., Angelici, R.: Equilibrium studies of copper(II) complexes of iminodiacetates with amino acid esters and kinetics of ester hydrolysis. Inorg. Chem. 8, 907–913 (1969)
Bydalek, T.J., Margerum, D.W.: Multidenate ligand kinetics. 1. Copper(II)- and ethylenediaminetetraacetatonickelate(II). J. Am. Chem. Soc. 83, 4326–4329 (1961)
Margerum, D.W., Bydalek, T.J.: Multidentate ligand kinetics. 5. Copper(II) and cyclohexylenediaminetetraacetatonickelate(II). Inorg. Chem. 2, 683–688 (1963)
Drumhiller, J.A., Montavon, F., Lehn, J.M., Taylor, R.W.: Complexation kinetics of highly substituted acyclic, monocyclic, and bicyclic tetraamines with copper(II) in basic aqueous-media. Inorg. Chem. 25, 3751–3757 (1986)
Liang, B.-F., Chung, C.-S.: Dissociation and isomerization kinetics of (meso-5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane)copper(ii) (blue) cation in strongly acidic aqueous media. Inorg. Chem. 20, 2152–2155 (1981)
Liang, B.-F., Chung, C.-S., Margerum, D.W.: Role of coordinated hydroxide ion in configurational conversions of tetraamine macrocyclic ligand complexes of copper(II). Inorg. Chem. 18, 2001–2007 (1979)
Acknowledgments
The financial support from Nouryon Surface Chemistry AB, Stenungsund, is gratefully acknowledged. The EXAFS measurements were performed at Stanford Synchrotron Radiation Laboratory, a national user facility operated by Stanford University on behalf of the U.S. Department of Energy, Office of Basic Energy Sciences. The SSRL Structural Molecular Biology Program is supported by the Department of Energy, Office of Biological and Environmental Research, and by the National Institutes of Health, National Center for Research Resources, Biomedical Technology Program.
Funding
Open Access funding provided by Swedish University of Agricultural Sciences.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
10953_2020_1050_MOESM1_ESM.doc
A summary of bond distances in reported in solid compounds containing bis(iminodiacetato/alkyl-N-iminoacetato)cuprate(II) complexes is given in Table S1. Table S2 summarizes all structure parameters in the refinements of the EXAFS data. Figure S1 shows the structure of zwitterionic n-dodecyl-N-iminodiacetic acid in solid state. Figure S2 shows the complex distribution functions for the copper(II)–methyl-N-iminodiacetate system in water, as function of copper(II) and ligand for the copper(II)–methyl- and n-hexyl-N-iminodiacetate systems. Figure S3 shows the potentiometric titration curves copper(II)–iminodiacetic acid hexyl-N-iminodiacetic acid at the composition 1:1. Figure S4 shows the calibration curves of the copper(II) n-alkyl-N-iminodiacetate complexes using the dye probe molecular absorption spectrophotometry method. Figure S5 shows the Fourier transforms of EXAFS data in Figure 3. The spetrophotometric method for determination of polyamine concentration using a colored metal complex as probe is presented in detail (DOCX 2512 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Häggman, L., Cassel, A. & Persson, I. Short Strong Hydrogen Bonds can Hinder Complex Formation: A Stability and Structure Study of Copper(II) Alkyl-N-iminodiacetic Acid Complexes in Aqueous Systems and Solid State. J Solution Chem 50, 203–219 (2021). https://doi.org/10.1007/s10953-020-01050-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-020-01050-7