Abstract
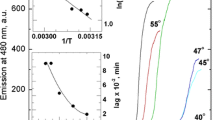
The mechanism of conversion of globular native proteins into amyloid fibrils represents one of the most attractive research topics in biophysics, because of its involvement in the development of severe pathologies and in various biotechnological processes. Aqueous medium properties, such as pH and ionic strength, as well as interactions with other species in solution, play a key role in tuning the fibrillization process. Here, we describe a comparative study of the influence of different ions from the Hofmeister series on the thermal unfolding and aggregation propensity of MNEI, a model protein, selected because of its tendency to form amyloid aggregates at acidic pH, even at temperatures well below its melting temperature. By selecting a temperature at which only negligible amounts of protein are unfolded, we have focused on the effect of ions on fibril formation. ThT fluorescence experiments indicated that all the salts examined increased the rate and the extent of fibrillization. Moreover, we found that anions, particularly sulfate, strongly influence the process, which instead is only marginally affected by different cations. Finally, a specific link to the chloride concentration was detected.





Similar content being viewed by others
References
Buell, A.K., Hung, P., Salvatella, X., Welland, M.E., Dobson, C.M., Knowles, T.P.J.: Electrostatic effects in filamentous protein aggregation. Biophys. J. 104, 1116–1126 (2013). https://doi.org/10.1016/j.bpj.2013.01.031
Marek, P.J., Patsalo, V., Green, D.F., Raleigh, D.P.: Ionic strength effects on amyloid formation by amylin are a complicated interplay among Debye screening, ion selectivity, and Hofmeister effects. Biochemistry 51, 8478–8490 (2012). https://doi.org/10.1021/bi300574r
Sirangelo, I., Iannuzzi, C.: The role of metal binding in the amyotrophic lateral sclerosis-related aggregation of copper–zinc superoxide dismutase. Molecules 22, 1429 (2017). https://doi.org/10.3390/molecules22091429
Koelsch, P., Motschmann, H.: An experimental route to Hofmeister. Curr. Opin. Colloid Interface Sci. 9, 87–91 (2004). https://doi.org/10.1016/j.cocis.2004.05.009
Lee, E., Choi, J.-H., Cho, M.: The effect of Hofmeister anions on water structure at protein surfaces. Phys. Chem. Chem. Phys. 19, 20008–20015 (2017). https://doi.org/10.1039/C7CP02826A
Násztor, Z., Dér, A., Bogár, F.: Ion-induced alterations of the local hydration environment elucidate Hofmeister effect in a simple classical model of Trp-cage miniprotein. J. Mol. Model. 23, 298 (2017). https://doi.org/10.1007/s00894-017-3471-0
Bye, J.W., Baxter, N.J., Hounslow, A.M., Falconer, R.J., Williamson, M.P.: Molecular mechanism for the Hofmeister effect derived from NMR and DSC measurements on barnase. ACS Omega. 1, 669–679 (2016). https://doi.org/10.1021/acsomega.6b00223
Imperatore, R., Vitiello, G., Ciccarelli, D., D’Errico, G.: Effects of salts on the micellization of a short-tailed nonionic ethoxylated surfactant: an intradiffusion study. J. Solution Chem. 43, 227–239 (2014). https://doi.org/10.1007/s10953-014-0133-z
Dér, A., Kelemen, L., Fábián, L., Taneva, S.G., Fodor, E., Páli, T., Cupane, A., Cacace, M.G., Ramsden, J.J.: Interfacial water structure controls protein conformation. J. Phys. Chem. B. 111, 5344–5350 (2007). https://doi.org/10.1021/jp066206p
Chaplin, M.F.: A proposal for the structuring of water. Biophys. Chem. 83, 211–221 (2000)
Salis, A., Ninham, B.W.: Models and mechanisms of Hofmeister effects in electrolyte solutions, and colloid and protein systems revisited. Chem. Soc. Rev. 43, 7358–7377 (2014). https://doi.org/10.1039/C4CS00144C
Kim, S.-H., Kang, C.-H., Kim, R., Cho, J.M., Lee, Y.-B., Lee, T.-K.: Redesigning a sweet protein: increased stability and renaturability. Protein Eng. 2, 571–575 (1989). https://doi.org/10.1093/protein/2.8.571
Tancredi, T., Iijima, H., Saviano, G., Amodeo, P., Temussi, P.A.: Structural determination of the active site of a sweet protein A 1H NMR investigation of pMNEI. FEBS Lett. 310, 27–30 (1992). https://doi.org/10.1016/0014-5793(92)81138-C
Esposito, V., Gallucci, R., Picone, D., Saviano, G., Tancredi, T., Temussi, P.A.: The importance of electrostatic potential in the interaction of sweet proteins with the sweet taste receptor. J. Mol. Biol. 360, 448–456 (2006). https://doi.org/10.1016/j.jmb.2006.05.020
Leone, S., Picone, D.: Molecular dynamics driven design of pH-stabilized mutants of MNEI, a sweet protein. PLoS ONE 11, e0158372 (2016). https://doi.org/10.1371/journal.pone.0158372
Leone, S., Pica, A., Merlino, A., Sannino, F., Temussi, P.A., Picone, D.: Sweeter and stronger: enhancing sweetness and stability of the single chain monellin MNEI through molecular design. Sci. Rep. 6, 34045 (2016). https://doi.org/10.1038/srep34045
Liu, Q., Li, L., Yang, L., Liu, T., Cai, C., Liu, B.: Modification of the sweetness and stability of sweet-tasting protein monellin by gene mutation and protein engineering. Biomed. Res. Int. 2016, e3647173 (2016). https://doi.org/10.1155/2016/3647173
Cai, C., Li, L., Lu, N., Zheng, W., Yang, L., Liu, B.: Expression of a high sweetness and heat-resistant mutant of sweet-tasting protein, monellin, in Pichia pastoris with a constitutive GAPDH promoter and modified N-terminus. Biotechnol. Lett. (2016). https://doi.org/10.1007/s10529-016-2182-4
Zheng, W., Yang, L., Cai, C., Ni, J., Liu, B.: Expression, purification and characterization of a novel double-sites mutant of the single-chain sweet-tasting protein monellin (MNEI) with both improved sweetness and stability. Protein Expr. Purif. 143, 52–56 (2018). https://doi.org/10.1016/j.pep.2017.10.010
Morris, J.A., Cagan, R.H.: Purification of monellin, the sweet principle of Dioscoreophyllum cumminsii. Biochim. Biophys. Acta. 261, 114–122 (1972). https://doi.org/10.1016/0304-4165(72)90320-0
Temussi, P.A.: Why are sweet proteins sweet? Interaction of brazzein, monellin and thaumatin with the T1R2–T1R3 receptor. FEBS Lett. 526, 1–4 (2002)
Spadaccini, R., Crescenzi, O., Tancredi, T., De Casamassimi, N., Saviano, G., Scognamiglio, R., Di Donato, A., Temussi, P.A.: Solution structure of a sweet protein: NMR study of MNEI, a single chain monellin. J. Mol. Biol. 305, 505–514 (2001). https://doi.org/10.1006/jmbi.2000.4304
Hobbs, J.R., Munger, S.D., Conn, G.L.: Monellin (MNEI) at 1.15 Å resolution. Acta Crystallogr. F 63, 162–167 (2007). https://doi.org/10.1107/s1744309107005271
Ghiso, J., Jensson, O., Frangione, B.: Amyloid fibrils in hereditary cerebral hemorrhage with amyloidosis of Icelandic type is a variant of gamma-trace basic protein (cystatin C). Proc. Natl. Acad. Sci. USA 83, 2974–2978 (1986)
Murzin, A.G.: Sweet-tasting protein monellin is related to the cystatin family of thiol proteinase inhibitors. J. Mol. Biol. 230, 689–694 (1993). https://doi.org/10.1006/jmbi.1993.1186
Konno, T.: Multistep nucleus formation and a separate subunit contribution of the amyloidgenesis of heat-denatured monellin. Protein Sci. 10, 2093–2101 (2001). https://doi.org/10.1110/ps.20201
Konno, T., Murata, K., Nagayama, K.: Amyloid-like aggregates of a plant protein: a case of a sweet-tasting protein, monellin. FEBS Lett. 454, 122–126 (1999). https://doi.org/10.1016/S0014-5793(99)00789-9
Rega, M.F., Di Monaco, R., Leone, S., Donnarumma, F., Spadaccini, R., Cavella, S., Picone, D.: Design of sweet protein based sweeteners: Hints from structure–function relationships. Food Chem. 173, 1179–1186 (2015). https://doi.org/10.1016/j.foodchem.2014.10.151
Esposito, V., Guglielmi, F., Martin, S.R., Pauwels, K., Pastore, A., Piccoli, R., Temussi, P.A.: Aggregation mechanisms of cystatins: a comparative study of monellin and oryzacystatin. Biochemistry 49, 2805–2810 (2010). https://doi.org/10.1021/bi902039s
Pica, A., Leone, S., Di, G., Donnarumma, F., Emendato, A., Rega, M.F., Merlino, A., Picone, D.: pH driven fibrillar aggregation of the super-sweet protein Y65R-MNEI: a step-by-step structural analysis. Biochim. Biophys. Acta 1862, 808–815 (2018). https://doi.org/10.1016/j.bbagen.2017.12.012
Patra, A.K., Udgaonkar, J.B.: Characterization of the folding and unfolding reactions of single-chain monellin: evidence for multiple intermediates and competing pathways. Biochemistry 46, 11727–11743 (2007). https://doi.org/10.1021/bi701142a
Kimura, T., Maeda, A., Nishiguchi, S., Ishimori, K., Morishima, I., Konno, T., Goto, Y., Takahashi, S.: Dehydration of main-chain amides in the final folding step of single-chain monellin revealed by time-resolved infrared spectroscopy. Proc. Natl. Acad. Sci. USA 105, 13391–13396 (2008). https://doi.org/10.1073/pnas.0801316105
Jha, S.K., Udgaonkar, J.B.: Direct evidence for a dry molten globule intermediate during the unfolding of a small protein. Proc. Natl. Acad. Sci. USA 106, 12289–12294 (2009). https://doi.org/10.1073/pnas.0905744106
Malhotra, P., Udgaonkar, J.B.: High-energy intermediates in protein unfolding characterized by thiol labeling under native like conditions. Biochemistry 53, 3608–3620 (2014). https://doi.org/10.1021/bi401493t
Goluguri, R.R., Udgaonkar, J.B.: Rise of the helix from a collapsed globule during the folding of monellin. Biochemistry 54, 5356–5365 (2015). https://doi.org/10.1021/acs.biochem.5b00730
Maity, H., Reddy, G.: Thermodynamics and kinetics of single-chain monellin folding with structural insights into specific collapse in the denatured state ensemble. J. Mol. Biol. (2017). https://doi.org/10.1016/j.jmb.2017.09.009
Aghera, N., Dasgupta, I., Udgaonkar, J.B.: A buried ionizable residue destabilizes the native state and the transition state in the folding of monellin. Biochemistry 51, 9058–9066 (2012). https://doi.org/10.1021/bi3008017
Spadaccini, R., Leone, S., Rega, M.F., Richter, C., Picone, D.: Influence of pH on the structure and stability of the sweet protein MNEI. FEBS Lett. 590, 3681–3689 (2016). https://doi.org/10.1002/1873-3468.12437
Leone, S., Sannino, F., Tutino, M.L., Parrilli, E., Picone, D.: Acetate: friend or foe? Efficient production of a sweet protein in Escherichia coli BL21 using acetate as a carbon source. Microb. Cell Factories. 14, 106 (2015). https://doi.org/10.1186/s12934-015-0299-0
Greenfield, N.J.: Using circular dichroism collected as a function of temperature to determine the thermodynamics of protein unfolding and binding interactions. Nat. Protoc. 1, 2527–2535 (2006). https://doi.org/10.1038/nprot.2006.204
Xue, C., Lin, T.Y., Chang, D., Guo, Z.: Thioflavin T as an amyloid dye: fibril quantification, optimal concentration and effect on aggregation. R. Soc. Open Sci. 4, 160696 (2017). https://doi.org/10.1098/rsos.160696
Sabate, R., Rodriguez-Santiago, L., Sodupe, M., Saupe, S.J., Ventura, S.: Thioflavin-T excimer formation upon interaction with amyloid fibers. Chem. Commun. 49, 5745–5747 (2013). https://doi.org/10.1039/C3CC42040J
Adrover, M., Mariño, L., Sanchis, P., Pauwels, K., Kraan, Y., Lebrun, P., Vilanova, B., Muñoz, F., Broersen, K., Donoso, J.: Mechanistic insights in glycation-induced protein aggregation. Biomacromolecules 15, 3449–3462 (2014). https://doi.org/10.1021/bm501077j
Mariño, L., Pauwels, K., Casasnovas, R., Sanchis, P., Vilanova, B., Muñoz, F., Donoso, J., Adrover, M.: Ortho-methylated 3-hydroxypyridines hinder hen egg-white lysozyme fibrillogenesis. Sci. Rep. (2015). https://doi.org/10.1038/srep12052
Stewart, K.L., Radford, S.E.: Amyloid plaques beyond Aβ: a survey of the diverse modulators of amyloid aggregation. Biophys. Rev. 9, 405–419 (2017). https://doi.org/10.1007/s12551-017-0271-9
Han, J.Y., Choi, T.S., Kim, H.I.: Molecular role of Ca2+ and hard divalent metal cations on accelerated fibrillation and interfibrillar aggregation of α-synuclein. Sci. Rep. 8, 1895 (2018). https://doi.org/10.1038/s41598-018-20320-5
Ramis, R., Ortega-Castro, J., Vilanova, B., Adrover, M., Frau, J.: A systematic DFT study of some plausible Zn(II) and Al(III) interaction sites in N-terminally acetylated α-synuclein. J. Phys. Chem. A 122, 690–699 (2018). https://doi.org/10.1021/acs.jpca.7b10744
Ramis, R., Ortega-Castro, J., Vilanova, B., Adrover, M., Frau, J.: Copper(II) binding sites in N-terminally acetylated α-synuclein: a theoretical rationalization. J. Phys. Chem. A 121, 5711–5719 (2017). https://doi.org/10.1021/acs.jpca.7b03165
Klement, K., Wieligmann, K., Meinhardt, J., Hortschansky, P., Richter, W., Fändrich, M.: Effect of different salt ions on the propensity of aggregation and on the structure of Alzheimer’s Aβ(1-40) amyloid fibrils. J. Mol. Biol. 373, 1321–1333 (2007). https://doi.org/10.1016/j.jmb.2007.08.068
Munishkina, L.A., Henriques, J., Uversky, V.N., Fink, A.L.: Role of protein–water interactions and electrostatics in alpha-synuclein fibril formation. Biochemistry 43, 3289–3300 (2004). https://doi.org/10.1021/bi034938r
Yeh, V., Broering, J.M., Romanyuk, A., Chen, B., Chernoff, Y.O., Bommarius, A.S.: The Hofmeister effect on amyloid formation using yeast prion protein. Protein Sci. 19, 47–56 (2010). https://doi.org/10.1002/pro.281
Arosio, P., Jaquet, B., Wu, H., Morbidelli, M.: On the role of salt type and concentration on the stability behavior of a monoclonal antibody solution. Biophys. Chem. 168–169, 19–27 (2012). https://doi.org/10.1016/j.bpc.2012.05.004
Yang, D.S., Yip, C.M., Huang, T.H., Chakrabartty, A., Fraser, P.E.: Manipulating the amyloid-beta aggregation pathway with chemical chaperones. J. Biol. Chem. 274, 32970–32974 (1999)
Sikkink, L.A., Ramirez-Alvarado, M.: Salts enhance both protein stability and amyloid formation of an immunoglobulin light chain. Biophys. Chem. 135, 25–31 (2008). https://doi.org/10.1016/j.bpc.2008.02.019
Zhang, Y., Cremer, P.S.: Chemistry of Hofmeister anions and osmolytes. Annu. Rev. Phys. Chem. 61, 63–83 (2010). https://doi.org/10.1146/annurev.physchem.59.032607.093635
Light, T.P., Corbett, K.M., Metrick, M.A., MacDonald, G.: Hofmeister ion-induced changes in water structure correlate with changes in solvation of an aggregated protein complex. Langmuir 32, 1360–1369 (2016). https://doi.org/10.1021/acs.langmuir.5b04489
Parsons, D.F., Boström, M., Nostro, P.L., Ninham, B.W.: Hofmeister effects: interplay of hydration, nonelectrostatic potentials, and ion size. Phys. Chem. Chem. Phys. 13, 12352–12367 (2011). https://doi.org/10.1039/c1cp20538b
Gokarn, Y.R., Fesinmeyer, R.M., Saluja, A., Razinkov, V., Chase, S.F., Laue, T.M., Brems, D.N.: Effective charge measurements reveal selective and preferential accumulation of anions, but not cations, at the protein surface in dilute salt solutions. Protein Sci. 20, 580–587 (2011). https://doi.org/10.1002/pro.591
Acknowledgements
The financial support of the “Fondazione con il Sud” (Grant No. 2011-PDR-19) is gratefully acknowledged. FD was recipient a fellowship financed by Regione Campania (POR Campania FSE 2014–2020).
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Donnarumma, F., Emendato, A., Leone, S. et al. Salt Modulated Fibrillar Aggregation of the Sweet Protein MNEI in Aqueous Solution. J Solution Chem 47, 939–949 (2018). https://doi.org/10.1007/s10953-018-0764-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-018-0764-6