Abstract
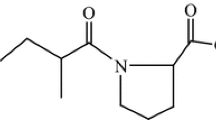
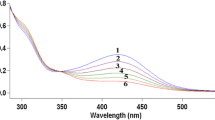
Kinetics of the oxidation of captopril by diperiodatoargentate(III) in aqueous alkaline medium was studied spectrophotometrically in the visible region. The kinetics of oxidation was found to be first order in diperiodatoargentate(III) and to have a fractional order in the captopril concentration. Added periodate, alkali and one of the reaction products, silver(I), showed retarding effects on the rate of reaction. The oxidation product of captopril was identified as captopril disulfide, which was characterized by its IR and LC–ESI–MS spectra. It was observed that the reaction products, silver(I) and captopril disulfide, interacted to yield a silver(I)–captopril disulfide complex and this was confirmed by its LC–ESI–MS spectrum. Based on the kinetics results and other parameters, a suitable mechanism for oxidation of captopril by diperiodatoargentate(III) has been proposed in which an intermediate complex between oxidant and substrate results from the preceding step before generation of a free radical in a slow step. The rate law was derived and reaction constants have also been determined. The active species of the silver(III)–periodate complex was determined to be monoperiodatoargentate(III).






Similar content being viewed by others
References
Cushman, D.W., Cheung, H.S., Sabo, E.F., Ondetti, M.A.: Design of potent competitive inhibitors of angiotensin-converting enzyme. Carboxyalkanoyl and mercaptoalkanoyl amino acids. Biochemistry 16, 5484–5491 (1977)
Ferguson, R.K., Brunner, H.R., Turini, G.A., Gavras, H., McKinstry, D.N.: A specific orally active inhibitor of angiotensin-converting enzyme in man. Lancet 1, 775–778 (1977)
Ondetti, M.A., Rubin, B., Cushman, D.W.: Design of specific inhibitors of angiotensin-converting enzyme: new class of orally active antihypertensive agents. Science 196, 441–444 (1977)
Rabenstein, D.L., Isab, A.A.: Conformational and acid–base equilibriums of captopril in aqueous solution. Anal. Chem. 54, 526–529 (1982)
Simona, C., Simona, C.P., Chis, V.: Captopril adsorption to silver nanostructures. Rom. J. Biophys. 17, 195–203 (2007)
Lin, B., Zhan, X.C., Li, L.L., Li, C.R., Qi, H.J., Tao, J.L.: Step nonisothermal method in kinetics in captopril oxidation under compressed oxygen. Yakugaku Zasshi 128, 617–624 (2008)
Khan, A.A.P., Mohd, A., Bano, S., Siddiqi, K.S.: Spectrophotometric interaction of the oxidation of captopril by hexacyanoferrate(III) in an alkaline medium: a kinetic and mechanistic approach. J. Sulfur Chem. 32, 427–434 (2011)
Jaiswal, P.K., Yadava, K.L.: Silver(III) as an oxidative titrant. Determination of some sugars, carboxylic acids and onorganic ions. Talanta 17, 236–238 (1970)
Rao, P.J.P., Sethuram, B., Rao, T.N.: Kinetics of oxidative deamination of some amino acids by diperiodatoargentate(III) in alkaline medium. React. Kinet. Catal. Lett. 29, 289–296 (1985)
Das, A., Mukhopadhay, S.: Kinetics of oxidation of glyoxylic acid by [ethylenebis(biguanide)]silver(III) in aqueous media. Polyhedron 23, 895–901 (2004)
Bandyopadhyay, P., Mukhopadhyay, S.: Kinetics of oxidation of hydroxylamine by [ethylenebis(biguanide)]silver(III) in aqueous media. Polyhedron 21, 1893–1898 (2002)
George, L., Atkinson, G.: The chemistry of argentic oxide. The formation of a silver(III) complex with periodate in basic solution. Inorg. Chem. 3, 1741–1743 (1964)
Mendham, J., Denney, R.C., Barnes, J.D., Thomas, M.J.K.: Vogel’s Text Book of Quantitative Analysis, 6th edn, p. 466. Pearson Education, Delhi (2003)
Lide, D.R.: CRC Handbook of Chemistry and Physics, 73rd edn. CRC Press, London (1992)
Kolthoff, I.M., Meehan, E.J., Carr, E.M.: Mechanism of initiation of emulsion polymerization by persulfate. J. Am. Chem. Soc. 75, 1439–1441 (1953)
Kirschenbaum, L.J., Rush, J.D.: Polypeptide complexes of silver(III). J. Am. Chem. Soc. 106, 1003–1010 (1984)
Cotton, F.A., Wilkinson, G.: Advanced Inorganic Chemistry—A Comprehensive Text, 6th edn. Wiley Interscience, New York (1996)
Banerjee, R., Das, K., Das, P., Dasagupta, S.: Kinetics of silver(I)-catalyzed oxidation of formic acid by the (ethylenebis(biguanidine))silver(III) cation in acid perchlorate media. Inorg. Chem. 28, 585–588 (1989)
Song, C., Chen, L., Shan, J.: Kinetics and mechanism of oxidation of leucine and alanine by Ag(III) complex in alkaline medium. Res. Lett. Chem. (2008). doi:10.1155/2008/786857
Jose, T.P., Angadi, M.A., Salunke, M.S., Tuwar, S.M.: Oxidation of atenolol by diperiodatoargentate(III) in aqueous alkaline medium—A multimechanistic reaction. Main Group Chem. 7, 109–122 (2008)
Angadi, M.A., Tuwar, S.M.: Oxidation of fursemide by diperiodatocuprate in aqueous alkaline medium—A kinetic study. J. Solution Chem. 39, 165–177 (2010)
Rabenstein, D.L., Theriault, Y.A.: Nuclear magnetic resonance study of the formation and conformational equilibria of symmetrical and mixed disulfides of captopril. Can. J. Chem. 63, 33–39 (1985)
Amis, E.S.: Solvent Effects on Reaction Rates and Mechanism. Academic Press, New York (1966)
Laidler, K.J.: Chemical Kinetics, 3rd edn. Pearson Education, New Delhi (2004)
Acknowledgments
The authors are grateful to the Principal, Karnatak Science College, Dharwad, Karnataka, India for providing the necessary facilities to carry out this work. They also thank the Raptakos Brett and Co., Microlabs Ltd. KLAB, Mumbai, India for providing the free sample of captopril.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Angadi, M.A., Tuwar, S.M. Mechanism of the Oxidation of Captopril by Diperiodatoargentate(III) in Aqueous Alkali: A Reaction Kinetics Study. J Solution Chem 44, 1844–1857 (2015). https://doi.org/10.1007/s10953-015-0379-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-015-0379-0