Abstract
This study uses density functional theory calculations to study the impact of pressure on the structural, elastic, and thermodynamic properties of CaRh2P2 and BaRh2P2 compounds. The equilibrium structural parameters closely match the experimental values. CaRh2P2 shows a greater reduction in lattice parameter “a” under pressure, while BaRh2P2 shows the opposite trend. The single-crystal elastic constants calculated for both compounds meet the criteria for mechanical stability under varying pressures up to 18 GPa. The study extends to polycrystalline elastic properties, including bulk modulus, shear modulus, Young’s modulus, Poisson’s ratio, and related properties, under pressures up to 18 GPa. The Pugh ratio, Cauchy pressure, and Poisson’s ratio indicate the ductile behavior of the title compounds. BaRh2P2 exhibits superconducting characteristics, unlike CaRh2P2, confirmed by interlayer PP bond length calculations. Debye’s quasi-harmonic model explores the temperature dependencies of various properties, with results that align well with elastic constant data, thus validating the results.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Advancements in modern technology and industry have seen an increasing reliance on superconducting materials in recent years. Notably, the discovery of high-temperature superconductors in 1986 sparked a revolutionary transformation, opening up possibilities for practical applications in diverse fields, including energy transfer, energy storage, and medical imaging [1, 2]. Among these materials, layered ternary intermetallic compounds with the ThCr2Si2-type (also known as 122-type) structure have been extensively investigated due to their intriguing and diverse physical phenomena [3]. Researchers have been particularly drawn to these materials because of their noteworthy properties, such as low-temperature superconductivity [4, 5], as well as the ability to exhibit high-temperature superconductivity under the influence of chemical doping and external pressure [6]. Additionally, these compounds exhibit other intriguing characteristics, such as heavy fermion behavior, unique magnetic ordering, and quantum criticality [3, 7,8,9]. The origins of the ThCr2Si2-type structure can be traced back to 1965 when Ban and Sikirica first reported it [10]. Subsequently, in 1996, Just and Paufler conducted an extensive geometric analysis of nearly 600 compounds with ThCr2Si2-type structure [11]. These compounds are typically represented by the chemical formula AB2X2, where A can be alkali, alkaline earth, or rare earth metals, B represents transition metals, and X includes elements from groups 13 to 15 of the periodic table [12, 13]. The 122-type ternary compounds exist in two distinct structures, depending on temperature conditions. The first structure, with a primitive tetragonal arrangement, is exemplified by CaBe2Ge2 (space group P4/nmm). The second structure, characterized by a body-centered tetragonal arrangement, is represented by ThCr2Si2 (space group I4/mmm) [14]. The ThCr2Si2-type structure corresponds to space group I4/mmm (no. 139) and has been identified in over seven hundred compounds [15]. The synthesis of iron-based superconductors with ThCr2Si2-type structure, such as AFe2As2 (A = Ca, Ba, Sr, Eu), showcasing high critical temperatures of up to 49 K, marked a remarkable breakthrough and played a pivotal role in advancing current technology [16]. However, due to challenges in manufacturing iron-based superconducting materials, researchers have directed their efforts toward exploring alternative ternary intermetallic ThCr2Si2-type materials with promising superconducting properties.
Hoffmann and Zheng [17] performed band structure calculations to elucidate the bonding and anti-bonding characteristics of the interlayer P-P bond in the ThCr2Si2 structure, which explains the noticeable variation in bond length. The length of the interlayer P-P bond has been identified as a crucial factor in the occurrence of superconductivity in the 122-type phosphides [18]. Theoretical considerations propose a critical interlayer P-P bond length of approximately 2.8 Å (dP−P ≈ 2.8 Å) for the formation of a covalent bond. Hence, to achieve a superconducting compound within the 122-type series, the interlayer P-P bond length needs to be greater than 2.8 Å [19]. Due to their intriguing superconductive properties at remarkably low temperatures (Tc below 3 K), ThCr2Si2-type compounds, such as CaPd2Ge2 (Tc = 1.98 K) [20], BaNi2P2 (Tc ≈ 3.0 K) [21], YIr2Si2 (Tc = 2.52 K) [22], BaIr2P2 (Tc = 2.1 K), BaRh2P2 (Tc = 1.0 K) [23], have attracted significant interest from researchers. It is evident that 122-type superconducting materials have played a crucial role in the advancement of modern technology. Therefore, the primary objective of this current study is to explore some fundamental physical properties of superconducting materials XRh2P2 (X = Ba, Ca). To achieve this, we have conducted ab initio calculations through the Density Functional Theory (DFT)-based pseudopotential plane wave (PP-PW) method. These calculations aim to determine the structural, elastic, and thermodynamic properties of the ternary phosphides BaRh2P2 and CaRh2P2. The paper is structured as follows: Section 2 provides a detailed explanation of the methodology and the specific calculation parameters employed. In Section 3, we present the analysis and discussion of the physical property results obtained from the calculations. Finally, Section 4 highlights the key findings of the study.
2 Calculational Approches and Settings
Our theoretical investigation was conducted using the pseudopotential plane wave (PP-PW) approach based on density functional theory (DFT) as implemented in the CASTEP program [24]. The exchange-correlation interactions were described using the PBEsol version of the generalized gradient approximation, known as GGA-PBEsol [25]. To model the Coulomb interactions between valence electrons (Ba-5s25p66s2, Rh-4d85s1, P-3s23p3, and Ca-3s23p64s2) and the ionic core, Vanderbilt-type ultrasoft pseudopotentials [26] were employed. For the basis set in the plane-wave approach, cut-off energy of 610 eV was chosen for BaRh2P2 and CaRh2P2. To sample the irreducible Brillouin zone, the Monkhorst-Pack scheme [27] was utilized with a grid of 8 × 8 × 11 k-points. The structural parameters were optimized using the BFGS (Broyden-Fletcher-Goldfarb-Shanno) technique [28]. Convergence tolerances were set as follows: the total energy convergence was within the limit of \(5.06 \times {10^{ - 6}}eV/atom\), the maximum Hellmann–Feynman force is within the limit of 0.01 eV / Å, the maximum displacement is within the limit of 5.0 × 10-4 Å, and the maximum stress is within the limit of \(0.0{\text{2 GPa}}\). The elastic constants (denoted as Cij) are ascertained through the meticulous execution of two distinct and specialized modes of finite lattice distortions. By subjecting the lattice to these deliberate deformations, the resultant strain-stress linear relationship is diligently analyzed and subsequently employed as the foundational basis for the derivation of the elastic constants [29]. Temperature and pressure dependencies of certain macroscopic thermodynamic parameters, such isobaric and isochoric heat capacities, thermal volume expansion coefficient and Debye temperature, of the studied materials were explored using the quasi-harmonic Debye model implemented in the GIBBS program [30].
3 Results and Discussion
3.1 Structural Properties
CaRh2P2 and BaRh2P2 materials adopt a tetragonal crystal structure known as the ThCr2Si2-type, characterized by the space group I4/mmm (No. 139) [31]. In the conventional unit cell (shown in Fig. 1), there are two formula units, housing a total of 10 atoms, while the primitive unit cell contains only one chemical formula, consisting of five atoms. The positions of these atoms are as follows: Ca (or Ba) atoms occupy the 2a site at coordinates (0,0,0), Rh atoms occupy the 4d site at coordinates (0,1/2,1/4), and P atoms are located at the 4e site with coordinates (0,0,zP). The internal parameter zP determines the z-coordinate of the P atom in the unit cell [18, 31]. The crystal structures of CaRh2P2 and BaRh2P2 can be conceptualized as alternating infinite layers. These layers consist of Rh atoms bonded with P atoms, forming RhP chains that are stacked along the crystallographic c-axis, the longest axis of the tetragonal lattice. These RhP chains are separated by the Ca (or Ba) atoms, which give rise to the overall structure. To determine the equilibrium lattice parameters a0 and c0, as well as the zP fractional coordinate that correspond to the minimum total energy of the XRh2P2 compounds (X = Ca, Ba), all free structural parameters, namely a, c and zP, have been fully relaxed. The resulting values for these parameters are presented in Table 1, along with previously reported values for the title compounds [18, 31] and for other compounds having the 122-type crystal structure [32,33,34,35,36]. The calculated equilibrium lattice parameters (a0 and c0) as well as the internal coordinate parameter zP for the XRh2P2 compounds, where X can be Ca or Ba, closely match the experimental counterparts available in the literature [18, 31]. This congruence between the computed and experimental data enhances the reliability and validity of the computational methods used to analyze the crystal structures of these materials. Such accurate alignment between theory and experiment is of significant importance in the field of solid-state physics and materials science, as it reaffirms the precision and predictive capability of computational techniques for investigating the structural properties of complex compounds like XRh2P2. The volume of the unit cell of BaRh2P2 is bigger than that of BaRh2P2, with values of V = 192.51 Å3 and V = 158.64 Å3, respectively. The sole distinction between these two compounds lies in the Ba and Ca atoms. Consequently, the observed variation in unit cell volume can be ascribed to the higher radius of the Ba atom compared to that of the Ca atom.
The length of the interlayer P-P bond plays a crucial role in determining the occurrence of superconductivity in 122-type phosphides [18]. This parameter, denoted as dP−P, serves as an indicator of the presence or absence of interlayer P-P covalent bonds. A critical length of the interlayer P-P bond of approximately 2.8 Å (dP−P ≈ 2.8 Å) is considered essential for the formation of interlayer P-P covalent bonds, and any value of dP−P greater than 2.8 Å indicates the absence of such a bond at the P-P interface, which is associated with the emergence of superconductivity. Upon calculating the interlayer P-P bond lengths for both CaRh2P2 and BaRh2P2 (as listed in Table 1), interesting findings emerge. For CaRh2P2, the computed value of dP−P is 2.23 Å, falling below the critical threshold of 2.8 Å. This indicates the formation of a P-P covalent bond in CaRh2P2. On the other hand, for BaRh2P2, the calculated dP−P value is 3.8 Å, surpassing the critical limit. Consequently, no interlayer P-P covalent bond is formed in BaRh2P2. These results provide an explanation for the differing superconducting behaviors observed in CaRh2P2 and BaRh2P2. As the interlayer P-P covalent bond is absent in BaRh2P2 due to its larger dP−P value, this compound exhibits superconductivity. In contrast, the presence of a P-P covalent bond in CaRh2P2, owing to its smaller dP−P value, prevents superconductivity from occurring. It is worth to remark that the distinctive difference between CaRh2P2 and BaRh2P2 is located in the Ba and Ca atoms. Thus the fact the interlayer P-P covalent bond in BaRh2P2 is bigger than that in CaRh2P2 may be attributed the fact that the atomic radius of Ba is larger than that of Ca.
We conducted an investigation into the pressure dependencies of the unit cell volume (V) and the lattice parameters (a and c). To analyze the behavior of these parameters under varying pressure conditions, we utilized normalized values. The normalized unit cell volume (V/V0) represents the ratio of the unit cell volume at a certain pressure (V) to its value at zero pressure (V0). Similarly, the normalized lattice parameters, a/a0 and c/c0, indicate the ratios of the lattice parameters (a and c) at pressure P to their respective values at zero pressure (a0 and c0). Second-order polynomials were employed to fit the variations of the normalized unit cell volume (V/V0) and the normalized lattice parameters (a/a0 and c/c0) under hydrostatic pressure. The fitting process allowed for a more precise representation of the relationships between the crystal lattice’s structural parameters and the applied pressure as follows:
From the data presented in Fig. 2a, it is evident that as the pressure increases, the normalized structural parameters diminish. In order to quantify this behavior, linear compressibility values along the 𝑎 and 𝑐 directions were estimated and found to be βa = \(2.35 \times {10^{-3}}\) GPa−1 (\(2.27 \times {10^{-3}}\) GPa−1) and βc = \(1.68 \times {10^{-3}}\) GPa−1 (\(5.49 \times {10^{-3}}\)GPa−1) for CaRh2P2 (BaRh2P2). Further examination of the data reveals that under the influence of pressure, a distinct behavior is observed in the variation of lattice parameters “a” and “c” for the CaRh2P2 compound compared to the BaRh2P2 compound. Specifically, for the CaRh2P2 compound, the lattice parameter “a” experiences a more pronounced reduction in response to applied pressure, whereas the lattice parameter “c” exhibits a comparatively less substantial decrease. This indicates an anisotropic response of the crystal lattice to pressure, with the “a” direction being more sensitive to compression compared to the “c” direction. In contrast, the BaRh2P2 compound demonstrates a converse trend. Here, under pressure, the lattice parameter “c” displays a more significant decrease compared to the lattice parameter “a” This signifies a differing response to pressure, with the “c” direction being more susceptible to compression than the “a” direction in this particular compound. It is important to mention that, despite the higher size of the unit cell of BaRh2P2 compared to CaRh2P2 at zero pressure, the lattice parameter “a” in BaRh2P2 is smaller than that in CaRh2P2, but the parameter “c” in BaRh2P2 is significantly larger than that in CaRh2P2. Consequently, the bond lengths along the a-axis in BaRh2P2 are shorter compared to those in CaRh2P2, indicating that the bonds along the a-axis in BaRh2P2 possess greater strength than the corresponding bonds in CaRh2P2. In contrast, the bonds along the c-axis in CaRh2P2 exhibit greater strength compared to the analogous bonds in BaRh2P2. The stronger the bond, the less sensitive it is to the pressure impact. This behavior may elucidate the observed inverse correlation between the lattice parameters “a” and “c” with respect to the pressure effect in CaRh2P2 and BaRh2P2.
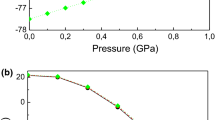
To estimate the bulk moduli (B) of the XRh2P2 compounds, where X can be Ca or Ba, two different approaches were employed for data fitting. Firstly, the total energy versus unit cell volume (E(V)) data was fitted to the third-order Birch-Murnaghan equation of state (BM-EOS) [37]. Secondly, the hydrostatic pressure versus unit cell volume (P(V)) data was fitted to the third-order Vinet equation of state (V-EOS) [38]. These fittings are illustrated in Fig. 2b and c. The obtained bulk modulus (B) values are presented in Table 1. Remarkably, there was a notable agreement between the values of B obtained through the BM-EOS and V-EOS approaches, thus affirming the consistency and reliability of the results. It is observed that the B value of BaRh2P2 is smaller than that of CaRh2P2, which is consistent with the established relationship: \(B\sim 1/V\), where V represents the unit cell volume.
a Pressure dependencies of a/a0, c/c0, and V/V0, and b total energy (Energy (eV)) as function of primitive cell volume (V) and c hydrostatic pressure (P) as function of primitive cell volume (V) for XRh2P2 (X = Ca, Ba). The subscript “0” denotes the parameter value at zero pressure. The symbols are the results obtained from the first principles calculations and the solid lines are the fits. b fit of the E–V data to the Birch–Murnaghan (BM) EOS, and c fit of the P–V data to Vinet EOS
To ensure the structural stability of the XRh2P2 compounds (where X can be either Ca or Ba), we performed an estimation of the formation enthalpy (∆H) using a specific expression [39, 40]:
In this context, \(E_{{Tot}}^{{XR{h_2}{P_2}}}\) represents the total energy of the primitive cell of XRh2P2 (X = Ca, Ba), and \(E_{{Tot}}^{{X(solid)}}\), \(E_{{Tot}}^{{Rh(solid)}}\), and \(E_{{Tot}}^{{P(solid)}}\) represent the total energies per atom of the solid state of the pure elements X (Ca or Ba), Rh, and P, respectively. nX, nRh, and nP are the numbers of X, Rh, and P atoms in the primitive cell, respectively. Applying this methodology, we obtained the enthalpies of formation given in Table 1 for XRh2P2 (X = Ca, Ba). Notably, upon examining Table 1, it becomes evident that the XRh2P2 compounds, whether X is Ca or Ba, exhibit negative formation enthalpies. This negative value indicates that these compounds are thermodynamically stable in their structural configuration.
3.2 Elastic Properties
3.2.1 Single-crystal Elastic Constants
Single-crystal elastic constants (Cij) play a crucial role in providing essential insights into various properties of materials, such as their bonding characteristics, how they respond to external stress, their mechanical stability, and overall stiffness [41]. In addition, the elastic constants are closely related to various fundamental physical properties, such as specific heat, melting point, Debye temperature, thermal expansion coefficient. In the context of a tetragonal system, there are six independent elastic constants that are of particular importance. These constants are denoted as C11, C12, C13, C33, C44, and C66. Table 2 presents the values of these six elastic constants for the XRh2P2 (X = Ca, Ba) tetragonal systems and those of some isostructural compounds [32, 35, 42]. Comparing these values with those from other related compounds helps in understanding the similarities and differences in the properties of these materials, thus contributing to the broader understanding of their behavior and applications. We can make some observations from the calculated elastic constants in XRh2P2 (X = Ca, Ba) compounds:
-
(i)
One notable observation from the calculated elastic constants (Cij) in XRh2P2 compounds (where X = Ca, Ba) is that C11 (which represents the linear compression resistance along the a-axis) is greater than C33 (which characterizes the linear compression resistance along the c-axis) in BaRh2P2. However, in CaRh2P2, C11 is smaller than C33. This finding suggests that the linear compressibility along the a-direction should be lower than that along the c-direction in BaRh2P2, while the opposite trend should be observed in the case of CaRh2P2. These results are consistent with the pressure dependence of lattice parameters “a” and “c” that we have already studied in Section 3.1.
-
(ii)
The Cij values show a decreasing trend from CaRh2P2 to BaRh2P2, demonstrating a decrease in Cij values as the unit cell volume increases.
-
(iii)
In the compound BaRh2P2, the value of C11 is greater than C33. However, in the compound CaRh2P2, the value of C11 is smaller than C33. This observation aligns with the controversies responses of “a” and “c” to changes in pressure in CaRh2P2 and BaRh2P2, as stated in Section 3.1.
-
(iv)
Another significant observation pertains to C44, which characterizes the material’s resistance against shear distortion in the (100) plane, and C66, which characterizes the resistance to shear in the <110> direction. The calculated values indicate that C44 is greater than C66, suggesting that XRh2P2 compounds (X = Ca, Ba) are more competent in resisting shear distortion along the <110> direction compared to the (100) plane [43].
-
(v)
The elastic constants C11 and C33 are somewhat larger than C44 and C66. This finding implies that XRh2P2 compounds (X = Ca, Ba) should be more resistant to unidirectional compression than to shear deformation.
-
(vi)
The Cauchy pressure (C12–C44) is an important indicator of material ductility or brittleness. When the Cauchy pressure is negative, the material tends to be brittle, whereas a positive value indicates ductility [43]. In the case of both XRh2P2 compounds (X = Ca, Ba), the Cauchy pressure (C12–C44) is positive, indicating that they are ductile materials.
-
(vii)
The linear compressibility along the a-direction (βa) and c-direction (βc) in a tetragonal crystal can be represented as follows [44]: βa = S11 + S12 + S13, and βc = S33 + 2S13, where Sij are the compliance constants The calculated values of βa and βc for CaRh2P2 (BaRh2P2) are found to be \(2.4 \times {10^{-3}}\) GPa−1 (\(2.5 \times {10^{-3}}\) GPa−1) and \(1.7 \times {10^{-3}}\) GPa−1 (\(5.9 \times {10^{-3}}\) GPa−1), respectively. These values are very close to the linear compressibilities obtained from the pressure dependence of the lattice parameters a and c studied in Section 3.1, and reflect well the fact that the linear compressibility along the a-direction is greater than that along the along the c-direction in CaRh2P2, while the compound BaRh2P2 exhibits an opposite trend. This close agreement supports the reliability of the calculated elastic constants.
-
(viii)
It is interesting to note that the values of the elastic constants (Cij) increase when the Ba atom in BaRh2P2 is replaced by the Ca atom, leading to a decrease in stiffness when transitioning from CaRh2P2 to BaRh2P2.
-
(ix)
In the realm of materials science, the critical parameter known as the melting temperature (Tm) assumes considerable significance, as it defines the temperature at which a given material undergoes a transformative shift from its solid state to a liquid state. The quantification of this pivotal parameter can be accomplished through the application of the following mathematical relationship [45]:
$$T_m=354+[4.5(2C_{11}+C_{33})/3]$$(4)The computed values od Tm for the considered compounds are presented in Table 2.
-
(x)
Lastly, it is crucial to consider the mechanical stability requirements at zero pressure. In this regard, the calculated values of the single-crystal elastic constants satisfy the required conditions for mechanical stability [46]:
$${C_{11}}>0;\; {C_{33}}>0;\; {C_{44}}>0;\; {C_{66}}>0;\; {C_{11}}+{C_{33}} - 2{C_{13}}>0;\; {C_{11}} - {C_{12}}>0;\; 2\left( {{C_{11}}+{C_{12}}} \right)+4{C_{13}}+{C_{33}}>0$$(5)
His indicates the mechanical stability of the XRh2P2 (X = Ca, Ba) compounds.
The graph presented in Fig. 3 illustrates the variations of Cij as a function of pressure within the range of 0 to 18 GPa. It is noteworthy that the calculated values of Cij at specific pressures (Cij(P)), namely 0, 3, 6, 9, 12, 15, and 18 GPa, fulfill the necessary conditions for mechanical stability under hydrostatic pressure [47, 48]:
In this context, \(C_{\alpha\alpha}=C_{\alpha\alpha}-P\;(\alpha=1,\;2,\;3,\;4,\;5,\;6)\), \(C_{12}=C_{12}+P\), and \(C_{13}=C_{13}+P\). This demonstrates that the materials XRh2P2 (where X can be either Ca or Ba) are mechanically stable under hydrostatic pressure. Furthermore, it has been observed that Cij exhibit a quadratic relationship with pressure. As the pressure increases, the elastic constants increase in accordance with the following quadratic polynomials:
3.2.2 Polycrystalline Elastic Moduli
The elastic properties of polycrystalline aggregates can be characterized by a pair of isotropic elastic moduli, namely the bulk and shear moduli (B and G). The polycrystalline bulk and shear moduli (B and G) can be determined from the single-crystal elastic constants (Cij) via the Voigt-Reuss-Hill approaches [49,50,51]. The Reuss [49] and Voigt [50] approximations are employed to obtain the extreme values of B (BR, BV) and G (GR, GV) for polycrystalline samples. The subscript “R” or “V” serves as a denotation that the adopted approximation pertains to the Reus or Voigt approximation, correspondingly. In the case of a tetragonal system, the polycrystalline elastic moduli BR, BV, GR, and GV are expressed as functions of Cij as follows [52]:
In this context, \(M=({C_{11}}+{C_{12}})+2{C_{33}} - 44{C_{13}}\), and \({C^2}=\left( {{C_{11}} - {C_{12}}} \right){C_{33}} - 2C_{{13}}^{2}\). According to the Hill approximation, the effective values of the bulk and shear moduli (BH and GH) are given by the arithmetic mean of the Voigt and Reuss limits [51]:
Another pair of polycrystalline elastic moduli, namely Young’s modulus (E) and Poisson’s ratio (σ), can be calculated from B and G using the following relationships:
Additionally, to quantify the hardness of a material, the Vickers hardness (HV) is commonly used as a powerful tool to describe the hardness of solids. Chen et al. [53, 54] proposed an expression for the Vickers hardness (HV) based on the obtained elastic moduli B and G, using the following relationship:
Here, \(K=G/B\). The predicted values of the polycrystalline moduli: BV, BR, GV, GR, GH, EH, σH, and HV, for the considered tetragonal crystals XRh2P2 (X = Ca, Ba) are listed in Table 3. This table also includes results from the literature for certain isostructural compounds [32, 37, 42]. From the data presented in Table 3, several significant conclusions can be drawn:
-
(i)
The bulk modulus (B) values obtained through the equation of state (EOS) fits for CaRh2P2 (B = 148.2 GPa) and BaRh2P2 (B = 91.8 GPa) closely align with those calculated from the single-crystal elastic constants, namely B = 149.6 GPa for CaRh2P2 and B = 93.9 GPa for BaRh2P2. This concordance between the two independent methods serves as strong evidence of the reliability and accuracy of the computed single elastic constants.
-
(ii)
The determination of a material’s brittleness or ductility holds considerable importance, as ductile materials are known for their ease of machinability and resilience to thermal shock. Poisson’s ratio (σ) and Pugh’s ratio (B/G) have proven to be effective indicators in distinguishing between ductile and brittle materials. When Poisson’s ratio is higher (or lower) than 0.26, the material is identified as ductile (or brittle) [43]. Similarly, if the B/G ratio exceeds (or is lower than) 1.75, the material exhibits ductile (or brittle) behavior [55, 56]. Based on the values listed in Table 3, it is evident that both CaRh2P2 and BaRh2P2 possess Poisson’s ratios greater than 0.26 and Pugh’s ratios larger than 1.75, pointing out their ductile behavior across various pressures (0, 3, 6, 9, and 18 GPa).
-
(iii)
A comparative analysis of the bulk modulus (B), shear modulus (G), and Young’s modulus (𝐸) between CaRh2P2 and BaRh2P2 reveals that the values of aforementioned elastic moduli of CaRh2P2 are consistently higher than those of BaRh2P2. This suggests that CaRh2P2 exhibits greater resistance to deformations, implying that the chemical bonds in CaRh2P2 possess a degree of strength superior to those in BaRh2P2.
-
(iv)
The Vickers hardness (HV) value in CaRh2P2 surpasses that of BaRh2P2, providing concrete evidence that CaRh2P2 possesses a higher hardness level than BaRh2P2.
-
(v)
Isotropic sound wave velocities, specifically the longitudinal, transverse, and mean sound wave velocities (denoted as VL, VT, and Vm), are fundamental and noteworthy physical parameters due to their intricate interrelation with various thermodynamic properties. These properties encompass the Debye temperature, thermal conductivity, heat capacity, and melting temperature. The quantities VL, VT, and Vm can be evaluated through explicit formulations using the isotropic bulk and shear moduli (designated as B and G, respectively), as articulated below:
$$V_T=\left(\frac G\rho\right)^{1/2};\;V_L=\left(\frac{3B+4G}{3\rho}\right)^{1/2};\;V_m=\left[\frac13\left(V_L^{-3}+2V_T^{-3}\right)\right]^{-1/3}$$(13)
The mass density ρ plays a pivotal role. The resulting VL, VT, and Vm values for the materials under scrutiny are documented within Table 3. The Debye temperature (symbolized as θD), an imperative thermodynamic parameter, is amenable to computation via the average sound wave velocity (Vm) in accordance with the ensuing expression [57,58,59]:
Here, the constants h, NA, kB, M, and n embody the Planck constant, Avogadro number, Boltzmann constant, molecular weight, and the count of atoms within the molecule, respectively. The computed θD values for the compounds of interest are thoughtfully juxtaposed with those belonging to some isostructural compounds [32, 35, 42], within the tabular arrangement denoted as Table 3. In the endeavor to comprehend the efficacy of heat propagation within materials, the parameter of thermal conductivity denoted as Kmin assumes paramount importance. This parameter’s significance is underscored by its intimate and direct correlation with high-temperature materials [60]. The lattice thermal conductivity Kmin for the XRh2P2 compounds where X stands for Ca and Ba has been calculated utilizing an established relationship [61]:
The plot shown in Fig. 3 illustrates the changes in polycrystalline elastic moduli, including bulk modulus (BH), shear modulus (GH), and Young’s modulus (EH), for both CaRh2P2 and BaRh2P2 as a function of applied pressure. To describe the relationship between these moduli and pressure, second-order polynomial equations were employed, which resulted in good fits to the calculated data. These polynomial equations effectively capture the variations of the moduli with increasing pressure.
Figure 4 shows that in the case of both examined compounds there exists a nearly linear augmentation of the longitudinal (VL), transversal (VT) and average (Vm) sound wave velocities with a rise in pressure. The alterations in VL, VT, and Vm exhibit a strong conformity with the mathematical representation of second-order polynomial equations:
The impact of pressure on key thermodynamic properties, namely the melting temperature (Tm), minimum thermal conductivity (Kmin), and Debye temperature (θD), for the compounds CaRh2P2 and BaRh2P2 is illustrated in Fig. 4. It is evident that these entire aforementioned parameters exhibit a consistent upward trend as pressure is incrementally augmented. The discernment is reinforced by the data in Table 3, wherein the Debye temperature for BaRh2P2 is notably lower compared to that of CaRh2P2. This discrepancy can be attributed to the inherent mechanical rigidity disparity between the two compounds, with CaRh2P2 demonstrating greater stiffness in comparison to BaRh2P2. The resultant polynomial expressions that represent the relationships for melting temperature (Tm), minimum thermal conductivity (Kmin), and Debye temperature (θD) pertaining to the XRh2P2 (X = Ca, Ba) compounds are enumerated as follows:
Calculated pressure-dependence of the transverse, longitudinal and average sound wave velocities (Vt, Vl and Vm) (a), Debye temperature (θD) (b), minimum thermal conductivity (Kmin) (c), and melting temperature (Tm) (d) for CaRh2P2 and BaRh2P2. The symbols are the calculated results, while the lines represent the fit curves
3.2.3 Elastic Anisotropy
The assessment of elastic anisotropy in crystals holds paramount significance due to its intimate relationship with various phenomena and material properties. Notably, it plays a pivotal role in understanding the development of plastic deformation in crystals [62], the stability and configuration of dislocations in materials, the texture of nanoscale shape-memory alloys [63], and the initiation of microcracks [64]. To quantitatively estimate the extent of elastic anisotropy in crystals, several metrics have been developed. In our study, we employed four distinct metrics to estimate the degree of elastic anisotropy exhibited by the compounds under investigation. By utilizing these metrics, we aim to gain comprehensive insights into the anisotropic behavior of the studied materials.
-
(i)
The evaluation of elastic anisotropy in crystals commonly relies on the calculation of percent anisotropy in shear (\({A_{Shear}}=\frac{{{G_V} - {G_R}}}{{{G_V}+{G_R}}} \times 100\)) and compression (\({A_{Comp}}=\frac{{{B_V} - {B_R}}}{{{B_V}+{B_R}}} \times 100\)) [65, 66]. Specifically, \({A_{Shear}}\) is defined as the ratio of shear modulus (G), while \({A_{Comp}}\) is the ratio of bulk modulus (B). These metrics serve as useful measures to quantify the extent of anisotropy in shear and compression within a crystal structure. The degree of anisotropy in shear and compression is reflected by the deviation of \({A_{Shear}}\) and \({A_{Comp}}\), respectively, from zero. Higher values of \({A_{Shear}}\) or \({A_{Comp}}\) indicate a more pronounced anisotropic behavior, while values closer to zero imply a more isotropic crystal. In Table 4, we present the calculated values of \({A_{Shear}}\) and \({A_{Comp}}\) for the studied tetragonal XRh2P2 (X = Ca, Ba) compounds. It is evident from these results that these materials exhibit a notable degree of anisotropy in shear and compression. This finding highlights the structural anisotropy in these crystals.
-
(ii)
To investigate the elastic anisotropy in crystals, researchers often employ the universal anisotropic index, denoted as AU, which is defined as \({A^U}=5\frac{{{G_V}}}{{{G_R}}}+\frac{{{B_V}}}{{{B_R}}} - 6\) [67, 68]. This index serves as a valuable metric for quantifying the degree of elastic anisotropy in a crystal structure. For isotropic crystals, the universal index equals zero, signifying a balanced distribution of elastic properties in all directions. However, for anisotropic crystals, the index deviates from zero, indicating the presence of varying degrees of anisotropy in the material’s response to shear and compression. In Table 4, we present the calculated values of the universal anisotropic index for the studied tetragonal XRh2P2 (X = Ca, Ba) compounds. The results confirm the presence of strong elastic anisotropy in both materials. This finding underscores the significant variation in their elastic properties along different crystallographic directions.
-
(iii)
The assessment of shear anisotropy factors A1 \(({A_1}={A_{\left\{ {100} \right\}}}=4{C_{44}}/\left( {{C_{11}}+{C_{33}} - 2{C_{13}}} \right))\) along the {100} shear planes and A3 \(({A_3}={A_{\left\{ {001} \right\}}}=2{C_{66}}/\left( {{C_{11}} - {C_{12}}} \right))\) along the {001} shear planes [69, 70] plays a crucial role in evaluating the degree of anisotropy in the bonding between atoms in different crystal planes. In the context of a tetragonal structure, the anisotropic shear factors 𝐴1 and 𝐴3 are expected to have values equal to unity for an isotropic crystal. Hence, any deviation from unity reflects the extent of elastic anisotropy. In Table 4, we present the calculated values of 𝐴1 and 𝐴3 for the title materials. The results indicate significant shear anisotropy along the {100} and {001} shear planes. This finding underscores the pronounced variation in bonding strengths and mechanical responses of atoms in different crystallographic directions.
-
(iv)
In a tetragonal system, the crystal direction-dependent Young’s modulus (E) and linear compressibility (β) can be expressed in the spherical coordinate system as follows [44]:
$$\left\{\begin{array}{l}\frac1E=\left(l_1^4+l_2^4\right)S_{11}+l_3^4S_{33}+2l_1^2l_2^2S_{12}+2\left(l_1^2l_3^2+2l_2^2l_3^2\right)S_{13}+\left(l_2^2l_3^2+l_1^2l_3^2\right)S_{44}+l_1^2l_2^2S_{66}\\\begin{array}{l}\beta=\left(S_{11}+S_{12}+S_{13}\right)-\left(S_{11}+S_{12}-S_{13}-S_{33}\right)l_3^2\end{array}\end{array}\right.$$(22)
Here, l1, l2, and l3 are the direction cosines, and Sij are the components of the compliance matrix. An elastic modulus isotropic with respect to crystal directions in 3D is represented by a closed surface that assumes a perfect spherical configuration. This is because the elastic modulus is the same in all directions for an isotropic material, and a sphere is the only shape that has the same radius in all directions. Therefore, the degree of deviation of the shape of this closed surface from its spherical shape reflects the degree of anisotropy of the represented elastic modulus. Figure 5 show the 3D contours of E and β as well as their cross-sections (2D contours) in the (001), (010), and (001) crystalline planes for the considered compounds. As can be seen, the 3D representations (2D representations) of the Young’s modulus deviate considerably from the spherical shape (circular form), indicating that this property is noticeably anisotropic for both studied compounds. In CaRh2P2 (CaRh2P2), the minimum value of E (Emin) is 184 GPa (94 GPa) along the [001] direction, and the maximum value (Emax) is 219 GPa (203 GPa) along the [110] direction. Similarly, the 3D-representations (2D-representations) of the linear compressibility of CaRh2P2 and BaRh2P2 deviate considerably from the spherical shape (circular form), indicating that this property is also noticeably anisotropic. In CaRh2P2 (BaRh2P2), the minimum value of β (βmin) is 0.0018 GPa−1 (0.0025 GPa−1) along the [001] direction (along any direction in the (001) plane), and the maximum value (βmax) is 0.0024 GPa−1 (0.0060 GPa−1) along any direction in the (001) plane (along the [001] direction) direction. This is consistent with the statement that has already been drawn from the elastic constants C11, and C33. The consistent difference between Emax (βmax) and Emin (βmin) indicates the strong anisotropy of the Young’s modulus (linear compressibility) in the considered compounds.
3.3 Thermodynamic Properties
The thermodynamic characteristics of the materials under investigation were subjected to comprehensive analysis through the utilization of the quasi-harmonic Debye approximation [71], and executed using the specialized computational framework known as the Gibbs software [30]. Within the scope of this particular investigation, a thorough exploration was conducted into the temperature-dependent behavior of several key material physical parameters. These physical parameters encompass the lattice parameter, bulk modulus, thermal expansion coefficient, heat capacity, and Debye temperature. The temperature range scrutinized spanned from absolute zero up to 600 K, and this analysis was conducted under controlled pressures held at fixed values of 0, 4, 8, 12, and 16.
Figure 6a illustrates the change in the lattice parameter “a” with change in temperature at the fixed pressures 0, 4, 8, 12, and 16 GPa. The lattice parameter increases as the temperature rises, while in contrast, it decreases with increasing pressure, proving the contrasting effects of temperature and pressure on the lattice parameter. Our computations indicate that at zero pressure and T = 300 K, the lattice parameter is 4.2698 Å for CaRh2P2, and 4.6112 Å for BaRh2P2.
Figure 6b illustrates the variation of the bulk modulus (B) as it responds to temperature alterations, all while being subjected to a series of unchanging pressures, namely 0, 4, 8, 12, and 16 GPa. Notably, the observed trend demonstrates a decline in the bulk modulus magnitude with the escalation of temperature. Furthermore, it becomes evident that an increase in pressure corresponds to an augmentation in the bulk modulus magnitude. Upon closer examination, it becomes evident that, under the conditions of zero pressure and a temperature of 300 K, the calculated bulk modulus values stand at 123.17 GPa for CaRh2P2 and 80.6 GPa for BaRh2P2. These numerical results distinctly indicate that CaRh2P2 exhibits a considerably greater resistance to deformation in comparison to BaRh2P2, implying a higher degree of structural rigidity and stability.
Thermal expansion (α), a phenomenon caused by the inherent mobility of the constituent atoms or molecules of a material, refers to the propensity of a substance to undergo volumetric expansion upon exposure to high temperatures. Figure 6c visually elucidates the nuanced trajectory of the coefficient of thermal expansion as a function of temperature and pressure changes. Notably, this diagram depicts a clear trend in which the coefficient of thermal expansion (α) experiences an accelerated increase as temperatures rises from zero to about 200 K. Beyond this temperature threshold, a more moderate and essentially linear progression characterizes his growth. Conversely, a noticeable decrease in the coefficient of thermal expansion is noticeable with increasing pressure, a trend evident in both composite materials. The coefficient of volumetric thermal expansion, evaluated at zero pressure and at a temperature of 300 K, amounts to \(5.98636\times10^{-5}\mathrm K^{-1}\) for CaRh2P2 and \(6.36372\times10^{-5}\mathrm K^{-1}\) for BaRh2P2, respectively.
Figure 6d and e shows the relationship between temperature and heat capacity, both at constant volume (CV) and constant pressure (CP), at the fixed pressures of 0, 4, 8, and 16 GPa. In the lower temperature steps, precisely covering the interval from 0 to 200 K, the values of CV and CP unquestionably follow a proportional relationship with the cubic power of temperature (T3). With increasing temperature, the increase in CV follows a gradual trajectory, gradually aligning with the eminent Dulong-Petit limit (\({\text{124}}{\text{0.66 J}}{\text{.mo}}{{\text{l}}^{-1}}{{\text{.K}}^{ - 1}}\)). In contrast, the CP constantly progresses in a linear fashion as the temperature increases. It is important to note that CV and CP tend to decrease as pressure increases. At a perfect intersection of zero pressure and a fixed temperature of 300 K, the estimated value of CV (CP) add up to 112.54 J.mol-1 .K-1 (118.76 J.mol-1 .K-1) for CaRh2P2 and \(118.19\;\mathrm J.\mathrm{mol}^{-1}\;.\mathrm K^{-1}\) \(\left(123.98\;\mathrm J.\mathrm{mol}^{-1\;}.\mathrm K^{-1}\right)\) for BaRh2P2.
The temperature dependence of the Debye temperature (θD) for the studied compounds at fixed pressures of 0, 4, 8, 12 and 16 GPa is illustrated in Fig. 6f. θD remains practically constant below 100 K, but then decreases gradually with increasing temperature. With increasing pressure, θD shows a noticeable increase, from \({\text{448}}.{\text{1 K}}\left( {{\text{322}}.{\text{6 K}}} \right)\) at zero pressure to \(550.8\;\mathrm K\;\left(423.1\mathrm K\right)\) at 16 GPa for CaRh2P2 (BaRh2P2). This increase in θD with pressure is due to the increase in the velocities of elastic waves, which in turn is caused by the increase in the interatomic forces. At zero pressure and 300 K, the approximate value of θD is 250 K for CaRh2P2 and 280 K for BaRh2P2. These values are in good agreement with the corresponding values derived from the elastic constants, which provides further confirmation of the reliability of the obtained results.
Temperature dependence of the lattice parameter (a) bulk modulus; B (b), thermal expansion coefficient; α (c), heat capacity at constant volume; CV (d) heat capacity at constant pressure; CP (d) and Debye temperature; θ (f) of CaRh2P2 and BaRh2P2 compounds at fixed pressures of 0, 4, 8, 12 and 16 GPa
4 Conclusion
The first-principles PP-PW method with the GGA-PBE exchange–correlation functional was used to calculate the effect of pressure on the structural, elastic, and thermodynamic properties of the tetragonal XRh2P2 (X = Ca, Ba) compounds. The main conclusions are as follows:
-
(i)
The computed ground state structural parameters are in good agreement with the experimental counterparts. The values obtained for the interlayer P-P bond distance reveal that BaRh2P2 is superconducting, while CaRh2P2 is not superconducting.
-
(ii)
The predicted values of the single-crystal elastic constants for the considered materials at zero-pressure as well as under hydrostatic pressure up to 18 GPa satisfy the mechanical stability conditions, and show high uniaxial elastic anisotropy.
-
(iii)
Pugh’s ratio, Cauchy pressure, and Poisson’s ratio indicate that XRh2P2 (X = Ca, Ba) are ductile materials. The XRh2P2 (X = Ca, Ba) compounds are expected to resist to shear distortion in the <110> more than in the (100) plane.
-
(iv)
The shear and bulk moduli (B, and G), Poisson’s ratio (σ), and Young’s modulus (E) increase with increasing pressure. The Vickers hardness, Debye temperature, sound wave velocity, melting temperature, and minimum thermal conductivity values were predicted in the pressure range from 0 to 18 GPa.
-
(v)
The temperature-dependency of the lattice parameter, bulk modulus, Debye temperature, volume thermal expansion coefficient, and isobar and isochoric heat capacities at the fixed pressures of 0, 4, 8, 12, and 16 GPa were successfully determined via the quasi-harmonic Debye model.
To conclude, there are no experimental studies regarding the thermodynamic and elastic properties of the tetragonal XRh2P2 (X = Ca, Ba) compounds to be compared to our results. Therefore, we expect that this study will stimulate further theoretical and experimental research activities.
Data Availability
No datasets were generated or analysed during the current study.
References
Felt, U., Nowotny, H.: Striking gold in the 1990s: the discovery of high-temperature superconductivity and its impact on the science system. Sci. Technol. Hum. Values 17(4), 506–531 (1992)
Sheahen, T.: Introduction to high-temperature superconductivity. Springer Science and Business Media (1994)
Steglich, F., Aarts, J., Bredl, C.D., Lieke, W., Meschede, D., Franz, W., Schäfer, H.: Superconductivity in the presence of strong pauli paramagnetism: CeCu2Si2. Phys. Rev. Lett. 43(25), 1892 (1979)
Ronning, F., Kurita, N., Bauer, E.D., Scott, B.L., Park, T., Klimczuk, T., Thompson, J.D.: The first order phase transition and superconductivity in BaNi2As2 single crystals. J. Phys. Condens. Matter 20(34), 342203 (2008)
Shein, I.R., Ivanovskii, A.L.: Electronic and structural properties of low-temperature superconductors and ternary pnictides ANi2Pn2 (A = Sr, Ba and P n = P, as). Phys. Rev. B 79(5), 054510 (2009)
Bauer, E.D., Ronning, F., Scott, B.L., Thompson, J.D.: Superconductivity in SrNi2As2 single crystals. Phys. Rev. B 78(17), 172504 (2008)
Trovarelli, O., Geibel, C., Mederle, S., Langhammer, C., Grosche, F.M., Gegenwart, P., Steglich, F.: YbRh2Si2: pronounced non-fermi-liquid effects above a low-lying magnetic phase transition. Phys. Rev. Lett. 85(3), 626 (2000)
Shelton, R.N., Braun, H.F., Musick, E.: Superconductivity and relative phase stability in 1: 2: 2 ternary transition metal silicides and germanides. Solid State Commun. 52(9), 797–799 (1984)
Shein, I.R., Ivanovskii, A.L.: Structural, elastic, electronic and magnetic properties of ThCr2Si2 from first-principles calculations. Solid State Commun. 151(17), 1165–1168 (2011)
Ban, Z., Sikirica, M.: The crystal structure of ternary silicides ThM2Si2 (M = cr, Mn, Fe, Co, Ni and Cu). Acta Crystallogr. A 18(4), 594–599 (1965)
Just, G., Paufler, P.: On the coordination of ThCr2Si2 (BaAl4-type compounds within the field of free parameters. J. Alloys. Compd. 232(1–2), 1–25 (1996)
Salma, M.U., Rahman, M.A.: Physical properties of ThCr2Si2-type Rh-based compounds ARh2Ge2 (A = ca, Sr, Y and Ba): DFT based first-principles investigation. Int J. Mod. Phys. B 32(32), 1850357 (2018)
Qin, M., Yang, C., Wang, Y., Yang, Z., Chen, P., Huang, F.: Synthesis, physical properties and electronic structure of Sr1 – xLaxCu2Pn2 (pn = P, as, sb). J. Solid State Chem. 187, 323–327 (2012)
Braun, H.F., Engel, N., Parthé, E.: Polymorphism and superconductivity of LaIr2Si2. Phys. Rev. B. 28(3), 1389 (1983)
Banu, I.S., Rajagopalan, M., Yousuf, M., Shenbagaraman, P.: Electronic and bonding properties of ANi2P2 (A = ca, Sr, Ba). J. Alloys Compd. 288(1–2), 88–95 (1999)
Anand, V.K., Kim, H., Tanatar, M.A., Prozorov, R., Johnston, D.C.: Superconducting and normal-state properties of APd2As2 (A = ca, Sr, Ba) single crystals. Phys. Rev. B 87(22), 224510 (2013)
Hoffmann, R., Zheng, C.: Making and breaking bonds in the solid state: The ThCr2Si2 structure. J. Phys. Chem. 89(20), 4175–4181 (2002)
Berry, N., Capan, C., Seyfarth, G., Bianchi, A.D., Ziller, J., Fisk, Z.: Superconductivity without Fe or Ni in the phosphides BaIr2P2 and BaRh2P2. Phys. Rev. B 79(18), 180502 (2009)
Foroozani, N., Lim, J., Schilling, J., Fotovat, R., Zheng, C., Hoffmann, R.: Hydrostatic high-pressure studies to 25 GPa on the model superconducting pnictide LaRu2P2. J. Phy. Conf. Ser. 500(3), 032007 (2014). IOP Publishing
Anand, V.K., Kim, H., Tanatar, M.A., Prozorov, R., Johnston, D.C.: Superconductivity and physical properties of CaPd2Ge2 single crystals. J. Phys. Condens. Matter 26(40), 405702 (2014)
Mine, T., Yanagi, H., Kamiya, T., Kamihara, Y., Hirano, M., Hosono, H.: Nickel-based phosphide superconductor with infinite-layer structure, BaNi2P2. Solid State Commun. 147(3–4), 111–113 (2008)
Vališka, M., Pospíšil, J., Prokleška, J., Diviš, M., Rudajevová, A., Sechovský, V.: Superconductivity in the YIr2Si2 and LaIr2Si2 polymorphs. J. Phys. Soc. Jpn. 81(10), 104715 (2012)
Hirai, D., Takayama, T., Higashinaka, R., Aruga-Katori, H., Takagi, H.: Superconductivity in layered pnictides BaRh2P2 and BaIr2P2. J. Phys. Soc. Jpn. 78(2), 023706–023706 (2009)
Clark, S.J., Segall, M.D., Pickard, C.J., Hasnip, P.J., Probert, M.I., Refson, K., Payne, M.C.: First principles methods using CASTEP. Z. Kristallogr. Cryst. Mater. 220(5–6), 567–570 (2005)
Perdew, J.P., Ruzsinszky, A., Csonka, G.I., Vydrov, O.A., Scuseria, G.E., Constantin, L.A., Burke, K.: Restoring the density-gradient expansion for exchange in solids and surfaces. Phys. Rev. Lett. 100(13), 136406 (2008)
Vanderbilt, D.: Soft self-consistent pseudopotentials in a generalized eigenvalue formalism. Phys. Rev. B 41(11), 7892 (1990)
Monkhorst, H.J., Pack, J.D.: Special points for Brillouin-Zone integrations. Phys. Rev. B 13(12), 5188 (1976)
Fischer, T.H., Almlof, J.: General methods for geometry and wave function optimization. J. Phys. Chem. 96(24), 9768–9774 (1992)
Kang, J., Lee, E.C., Chang, K.J.: First-principles study of the structural phase transformation of hafnia under pressure. Phys. Rev. B. 68(5), 054106 (2003)
Blanco, M.A., Francisco, E., Luana, V.: GIBBS: Isothermal-isobaric thermodynamics of solids from energy curves using a quasi-harmonic debye model. Comput. Phys. Commun. 158(1), 57–72 (2004)
Wurth, A., Johrendt, D., Mewis, A., Huhnt, C., Michels, G., Roepke, M., Schlabitz, W.: Über Den Einfluß Von Temperatur, Druck und substitution auf die Kristallstruktur Von ARh2P2 (A = ca, Sr, Eu, Ba). Z. Anorg. Allg. Chem. 623(9), 1418–1424 (1997)
Radjai, M., Bouhemadou, A.: Physical properties of BaRu2X2 (X = P, as) superconducting compound sunder pressure effects: Ab initio study. Physica C. (Amsterdam, Neth.) 604, 1354176 (2023)
Wenski, G., Mewis, A.: BaAl4-Strukturvarianten bei ARu2X2 (A = ca, Sr, Ba, Eu; X = P, as) und APt2P2 – x (A = ca, Eu)/BaAl4-Denvative structures of ARu2X2 (A = ca, Sr, Ba. Eu; X = P, as) and of APt2P2 – x (A = ca, Eu). Z. Naturforsch. B 41(1), 38–43 (1986)
Sasmal, K., Lv, B., Lorenz, B., Guloy, A.M., Chen, F., Xue, Y.Y., Chu, C.W.: Superconducting Fe-based compounds (A1 – x Srx) Fe2As2 with A = K and Cs with transition temperatures up to 37 K. Phys. Rev. Lett. 101(10), 107007 (2008)
Radjai, M., Bouhemadou, A., Bitam, T.: First-principles investigation of the structural, elastic and thermodynamic properties of CaRu2X2 (X = P, As) under pressure. J. Supercond. Novel. Magn. 35(9), 2531–2544 (2022)
Jeitschko, W., Glaum, R., Boonk, L.: Superconducting LaRu2P2 and other alkaline earth and rare earth metal ruthenium and osmium phosphides and arsenides with ThCr2Si2 structure. J. Solid State Chem. 69(1), 93–100 (1987)
Birch, F.: Finite elastic strain of cubic crystals. Phys. Rev. 71(11), 809 (1947)
Vinet, P., et al.: Compressibility of solids. J. Geophys. Res. Solid Earth 92(B9), 9319–9325 (1987)
Radjai, M., Maouche, D., Guechi, N., Cheddadi, S., Kechidi, Z.: Investigation of structural and elastic properties of monoclinic Ba2P7X (X = Cl, Br, I) Zintl Salts compounds. arXiv preprint arXiv:1910.00915 (2019)
Chaba Mouna, S., Radjai, M., Bouhemadou, A., Houatis, D., Allali, D., Essaoud, S., Bin-Omran, S.: Structural, elastic, and thermodynamic properties of BaXCl3 (X = Li, Na) perovskites under pressure effect: ab initio exploration. Phys. Scr. 98(6), 065949 (2023)
Westbrook, J.H.: Intermetallic compounds, structural applications of, Vol. 3 Wiley (2000)
Rehman, J.U., Usman, M., Tahir, M.B., Hussain, A., Rashid, M.: Investigation of structural, electronics, optical, mechanical and thermodynamic properties of YRu2P2 compound for superconducting application. J. Supercond. Novel Magn. 34(12), 3089–3097 (2021)
Hadi, M.A., Ali, M.S., Naqib, S.H., Islam, A.K.: New ternary superconducting compound LaRu2As2: physical properties from density functional theory calculations. Chin. Phys. B 26(3), 037103 (2017)
Nye, J.F.: Physical properties of crystals: their representation by tensors and matrices. Oxford University Press (1985)
Alouani, M., Albers, R.C., Methfessel, M.: Calculated elastic constants and structural properties of Mo and MoSi2. Phys. Rev. B 43(8), 6500 (1991)
Born, M., Huang, K., Lax, M.: Dynamical theory of crystal lattices. Am. J. Phys. 23(7), 474–474 (1955)
Wu, Z.J., Zhao, E.J., Xiang, H.P., Hao, X.F., Liu, X.J., Meng, J.: Crystal structures and elastic properties of superhard IrN2 and IrN3 from first principles. Phys. Rev. B 76(5), 054115 (2007)
Sin’Ko, G.V., Smirnov, N.A.: Ab initio calculations of elastic constants and thermodynamic properties of bcc, fcc, and hcp Al crystals under pressure. J. Phys. Condens. Matter 14(29), 6989 (2002)
Reuss, A., Angew, Z.: Math. Mech. 9, 5566 (1929)
Voigt, W.: Lehrbuch der Kristallphysik (Leipzig: Teubner). Adv. Earth Science 1, 1–978 (1928)
Hill, R.: The elastic behaviour of a crystalline aggregate. Proc. Phys. Soc. A 65(5), 349 (1952)
Bedjaoui, A., Bouhemadou, A., Bin-Omran, S.: Structural, elastic and thermodynamic properties of tetragonal and orthorhombic polymorphs of Sr2GeN2: an ab initio investigation. High Press. Res. 36(2), 198–219 (2016)
Chen, X.Q., Niu, H., Franchini, C., Li, D., Li, Y.: Hardness of T-carbon: density functional theory calculations. Phys. Rev. B 84(12), 121405 (2011)
Chen, X.Q., Niu, H., Li, D., Li, Y.: Modeling hardness of polycrystalline materials and bulk metallic glasses. Intermetallics 19(9), 1275–1281 (2011)
Pugh, S.F.: XCII. Relations between the elastic moduli and the plastic properties of polycrystalline pure metals. Lond. Edinb. Dublin Philos. Mag. & J. Sci. 45(367), 823–843 (1954)
Radjai, M., Bouhemadou, A., Bin-Omran, S.: Ab initio study of pressure dependence of the structural, elastic and thermodynamic properties of AlXY3 (X = B, C). Phase Transit. 96(1), 1–15 (2023)
Anderson, O.L.: A simplified method for calculating the Debye temperature from elastic constants. J. Phys. Chem. Solids 24(7), 909–917 (1963)
Radjai, M., Bouhemadou, A., Maouche, D.: Structurlastic, electronic and optical properties of the half-Heusler ScPtSb and YPtSb compounds under pressure. arXiv preprint arXiv:211209940 (2021)
Kanchana, V., Vaitheeswaran, G., Svane, A., Delin, A.: First-principles study of elastic properties of CeO2, ThO2 and PoO2. J. Phys. Condens. Matter 18(42), 9615 (2006)
Karaca, E., Karadaǧ, S., Tütüncü, H.M., Srivastava, G.P., Uǧur, Ṣ: First-principles investigation of superconductivity in the body-centred tetragonal. Phil. Mag. 96(19), 2059–2073 (2016)
Clarke, D.R.: Materials selection guidelines for low thermal conductivity thermal barrier coatings. Surf. Coat. Technol. 163, 67–74 (2003)
Li, J., Van Vliet, K.J., Zhu, T., Yip, S., Suresh, S.: Atomistic mechanisms governing elastic limit and incipient plasticity in crystals. Nature 418(6895), 307–310 (2002)
Lloveras, P., Castán, T., Porta, M., Planes, A., Saxena, A.: Influence of elastic anisotropy on structural nanoscale textures. Phys. Rev. Lett. 100(16), 165707 (2008)
Tvergaard, V., Hutchinson, J.W.: Microcracking in ceramics induced by thermal expansion or elastic anisotropy. J. Am. Ceram. Soc. 71(3), 157–166 (1988)
Huang, B., Duan, Y.H., Hu, W.C., Sun, Y., Chen, S.: Structural, anisotropic elastic and thermal properties of MB (M = Ti, Zr and Hf) monoborides. Ceram. Int. 41(5), 6831–6843 (2015)
Li, R.Y., Duan, Y.H.: Anisotropic elastic properties of MB (M = Cr, Mo, W) monoborides: a first-principles investigation. Phil. Mag. 96(10), 972–990 (2016)
Ozisik, H.B., Colakoglu, K., Deligoz, E.: First-principles study of structural and mechanical properties of AgB2 and AuB2 compounds under pressure. Comput. Mater. Sci. 51(1), 83–90 (2012)
Radjai, M., Guechi, N., Maouche, D.: An ab initio study of structurlastic and electronic properties of hexagonal MAuGe (M = Lu, Sc) compounds. arXiv preprint arXiv:2103.15579 (2021)
Mo, Y., Pang, M., Yang, W., Zhan, Y.: Effects of alloying elements on structural, electronic and mechanical properties of AlSc2 by first-principles calculations. Comput. Mater. Sci. 69, 160–167 (2013)
Wu, M.M., Wen, L., Tang, B.Y., Peng, L.M., Ding, W.J.: First-principles study of elastic and electronic properties of MgZn2 and ScZn2 phases in Mg–Sc–Zn alloy. J. Alloys Compd. 506(1), 412–417 (2010)
Blanco, M.A., Pendás, A.M., Francisco, E., Recio, J.M., Franco, R.: Thermodynamical properties of solids from microscopic theory: applications to MgF2 and Al2O3. J. Mol. Struct. (Thoechem) 368, 245–255 (1996)
Acknowledgements
The author S. Bin-Omran acknowledges the Researchers Supporting Project number RSP2024R82, King Saud University, Riyadh, Saudi Arabia.
Funding
This research was supported by Researchers Supporting Project Number (RSP2024R82), King Saud University, Riyadh, Saudi Arabia.
Author information
Authors and Affiliations
Contributions
Authors’ contributions: M.S: Writing - original draft. M.R: Conceptualization, Formal analysis. A.B: Visualization, Writing - review, editing, Validation, Methodology. Y.I.B: Review, Editing. S.C: Prepared figures, Writing. S.B: Methodology. All authors carefully reviewed the manuscript in all its versions.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Slimani, M., Radjai, M., Bouhemadou, A. et al. Ab Initio Prediction of the Structural, Elastic and Thermodynamic Properties Under Hydrostatic Pressure of the Ternary Tetragonal Phosphides XRh2P2 (X = Ca, Ba) for Superconducting Application. J Supercond Nov Magn (2024). https://doi.org/10.1007/s10948-024-06754-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10948-024-06754-1