Abstract
In this work, the Fe60Pt40 nanoparticles were synthesized through the chemical reduction method by using the Fe(CO)5 and the Pt(acac)2 as the metallic precursor. The particle size of the synthesized FePt nanoparticles was controlled by adjusting the reaction conditions involving of the Fe(CO)5-injecting temperature and the soak time of reaction. The structure, morphology, and magnetic properties of the synthesized FePt nanoparticles were characterized by XRD, TEM, and vibrating sample magnetometer (VSM), respectively. The synthesized nanoparticles exhibit uniform size and good dispersity. The magnetization behaviors indicate that the nanoparticles present the core-shell magnetic structure including the ferromagnetic core part and the spin-disorder shell part. There exists a spin pinning effect of the shell part on the core part. The increase of particle size weakens the spin pinning effect.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Nowadays, the magnetic nanoparticles (NPs) including the FePt and CoPt NPs have attracted considerable attention due to their peculiar magnetic properties such as high magnetic coercive force, high saturated magnetization, and the effect of magnetic hyperthermia. Among them, the FePt nanoparticles are mostly studied because of their potential applications in high-performance permanent magnets, high-density data storage, biomedicine, novel inks for inkjet printer, and so on [1,2,3,4,5]. The FePt nanoparticles present different crystalline structures rest with the synthetic method and synthesis condition. The FePt nanoparticles with the different crystalline structures show the different magnetic properties and are applicable in different fields. The as-grown FePt nanoparticles mostly have a face-centered cubic structure (fcc-FePt). The fcc-FePt shows superparamagnetic behavior [1]. When the atomic ratio between Fe and Pt is approximated to 1, the FePt nanoparticles with the tetragonal structure, named as L10-FePt or fct-FePt, could be synthesized. In the fct-FePt, Fe and Pt place the ordered arrangement [6, 7]. Because of this chemical order and the strong spin-orbit coupling, the L10-FePt possesses high magnetic coercive force at room temperature, which makes the L10-FePt applied in the field of high-density data storage. Additionally, there exists a third ordered cubic crystalline structure in the FePt NPs named as L12-FePt3. The L12-FePt3 NPs show superparamagnetism ascribing to the high magneto-crystalline anisotropy energy and could be used in biomedicine [8,9,10,11].
The FePt nanoparticles are generally synthesized by the chemical reduction method. The crystalline structure of the synthesized FePt nanoparticles is related with the reaction kinetics in the synthesis process which is controlled by the synthesis condition including the mole ratio between Fe and Pt, the type and quantity of surfactants, solvents and reducing reagent, reaction temperature, and the time of keeping reaction temperature. The fcc-FePt phase is easily synthesized on account of the low reaction kinetic energy. The L10-FePt is also a relatively stable phase. Furthermore, the L10-FePt nanoparticles were mostly studied because of their highly potential application in the high-density data storage [11]. Few works were focused on the fcc-FePt nanoparticles [6]. However, the magnetic properties of the fcc-FePt nanoparticles still need to be further clarified as the particle size decreases to the nano-scale. In this work, we investigated the magnetic properties of the fcc-FePt nanoparticles with the varied nanoparticle size. The interesting spin pinning effect is found in the fcc-FePt nanoparticles with the core-shell magnetic structure. This pinning effect is adjusted by the particle size of nanoparticles.
2 Experimental
2.1 Synthesis
The chemical reduction method was employed to synthesize the FePt nanoparticles. In this method, Fe(CO)5 was used as the metallic precursor of Fe [12]. We used the poly(N-vinyl-2-pyrrolidone) (PVP) instead of the low molecular weight ligands as the stabilizer and surface dispersant to get the FePt nanoparticles without post-annealing in one of the ways [13, 14]. The synthesis of FePt nanoparticles was implemented in an inert gas of nitrogen in the whole process. At first, the Pt(acac)2 (0.3933 g, 1.0 mmol) was added into the four-neck flask. Then, the benzyl ether, oleyl amine (OAm; 2.0 mL), and oleic acid (OA; 1.0 mL) were pipetted in the flask successively. In this method, Pt(acac)2 and Fe(CO)5 were used as the metallic precursors. Benzyl ether and OAm were used as the solvents. The OA and OAm were used as the surfactants. Subsequently, the mixture was stirred adequately and degassed at constant temperature of 100 °C for 90 min. Then in the following process, the mixture was under the continuous nitrogen atmosphere. In the meantime, the Fe(CO)5 (0.3 mL) was injected into the mixture. Then the mixed solution was heated to 240 °C and soaked at this temperature for 1 or 2 or 3 h. After that, the mixture was cooled to the room temperature spontaneously [15,16,17]. The experimental product was washed by hexane and ethanol thoroughly. To make the product more pure, the product was centrifuged several times and dissolved into the ethanol to store. In other synthesizing processes, in order to control the size of the FePt nanoparticles, the reaction conditions were adjusted. In this work, the temperature of the Fe(CO)5 injecting and the time of reaction were varied in the different reaction.
2.2 Characterization
The structure and morphology of the FePt nanoparticles were explored by the X-ray diffraction (XRD) and the transmission electron microscopy (TEM), respectively. The element analysis was carried out through the energy-dispersive X-ray spectroscopy (EDX). The magnetic properties of FePt nanoparticles were measured by vibrating sample magnetometer (VSM) [18,19,20].
3 Results and Discussion
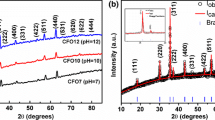
Figure 1 shows the XRD pattern of one of the typical synthesized FePt nanoparticles. The peaks of the XRD pattern can be indexed under the fcc structure. There are no other impurity peaks. Therefore, the product is the pure fcc-FePt without other impurities.
In this work, the FePt nanoparticles with different average particle sizes were achieved. Figure 2 exhibits the TEM images of the FePt nanoparticles with the average particle diameters of 2, 3, 4, and 5 nm. It can be found from the TEM image that the sample has a good dispersion and the particle size is uniform.
To determine the composition of the products, we investigate the composition of the synthesized FePt NPs. Figure 3 shows the EDX pattern of the typical FePt nanoparticles with the average particle diameters of 4 nm. Only the Fe and Pt peaks appear in the EDX spectra. This also confirms the synthesis of the pure FePt nanoparticles. Through calculation of the EDX spectra, the molar rate of Fe and Pt is obtained to be 60:40% (inset of Fig. 3). Therefore, the synthesized FePt NPs are Fe60Pt40. In this work, we controlled the same molar rate of the precursors Fe(CO)5 and Pt(acac)2 in the different reactions and obtained the same composition of the FePt NPs.
The magnetic properties of the FePt nanoparticles with different average particle sizes were measured at 5 K. Figure 4 shows the field dependence of magnetization for the FePt nanoparticles with the average particle diameters of 2, 3, 4, and 5 nm at 5 K. The 2-nm FePt nanoparticles show the magnetic hysteresis loop at 5 K. The shape of magnetic hysteresis is not normal and like dumbbell with the coercive field of 0 Oe. When the particle size increases, the phenomenon of the magnetic hysteresis loop weakens and disappears for the 4- and 5-nm FePt nanoparticles. The phenomena are ascribed to the core-shell magnetic structure in the FePt nanoparticles. At low temperature of 5 K, spins in the core region align the ferromagnetic order and spins in the shell region present disorder due to crystalline mismatch. In the field-magnetizing process, when the magnetic field is zero, the magnetizations of all nanoparticles direct randomly due to the good diversity of the nanoparticles. Thus, the whole magnetization is zero and the coercive field is 0 Oe. When the field increases, the spins in ferromagnetic core region will rotate to the direction of the external field whereas the spins in the shell region persist disorder. Furthermore, the spin-disorder shell generates the pinning effect on the ferromagnetic core region. This pinning effect is the origin of magnetic hysteresis. When the size of nanoparticle increases, the core part increases and the volume rate between the shell and core region decreases, which leads to the weakness and disappearance of the pinning effect. Thus, the spin pinning effect could be adjusted by the particle size.
4 Conclusion
The homogeneous Fe60Pt40 nanoparticles were successfully synthesized through the chemical reduction method. The particle size of the synthesized FePt nanoparticles was controlled by adjusting the reaction conditions. The synthesized nanoparticles show uniform size and good dispersity. The magnetization behaviors indicate that the nanoparticles present the core-shell magnetic structure including the ferromagnetic core part and the spin-disorder shell part. There exists the spin pinning effect of the shell part on the core part when the external magnetic field increases. The increase of particle size weakens the spin pinning effect.
References
Sasaki, Y., Mizuno, M., Yu, A.C.C., Inoue, M., Yazawa, K., Ohta, I., et al.: Crystallographic structures and magnetic properties of L10-type FePt nanoparticle monolayered films stabilized on functionalized surfaces. J. Magn. Magn. Mater. 282, 122–126 (2004)
Srivastava, S., Gajbhiye, N.S.: Exchange coupled L10-FePt/fcc-FePt nanomagnets: synthesis, characterization and magnetic properties. J. Magn. Magn. Mater. 401, 969–976 (2016)
Sato, K., Jeyadevan, B., Tohji, K.: Preparation and properties of ferromagnetic FePt dispersion. J. Magn. Magn. Mater. 289, 1–4 (2005)
Wu, L., Mendoza-Garcia, A., Li, Q., Sun, S.: Organic phase syntheses of magnetic nanoparticles and their applications. Chem. Rev. 116, 10473–10512 (2016)
Liu, Y., Li, D.G., Sun, S.S.: Pt-based composite nanoparticles for magnetic, catalytic, and biomedical applications. J. Mater. Chem. 21, 12579–12587 (2011)
Sun, S.: Recent advances in chemical synthesis, self-assembly, and applications of FePt nanoparticles. Adv. Mater. 18, 393–403 (2006)
Wang, J.P., Magnetic, F.P.: Nanoparticles and their assembly for future magnetic media. P Ieee. 96, 1847–1863 (2008)
Julia, L., Ingo, O., Karl-Hartmut, M., Oliver, G., Manuel, R., Manfred, W., Ludwig, S.: Magnetocrystalline anisotropy in L10 FePt and exchange coupling in FePt/Fe3 Pt nanocomposites. J. Phys. Condens. Matter. 17, 4157–4170 (2005)
Maat, S., Hellwig, O., Zeltzer, G., Fullerton, E.E., Mankey, G.J., Crow, M.L., Robertson, J.L.: Antiferromagnetic structure of FePt3 films studied by neutron scattering. Phys. Rev. B. 63, 134426 (2001)
Heitsch, A.T., Lee, D.C., Korgel, B.A.: Antiferromagnetic single domain L12 FePt3 nanocrystals. J. Phys. Chem. C. 114, 2512–2518 (2010)
Wang, B., Berry, D.C., Chiari, Y., Barmak, K.: Experimental measurements of the heats of formation of Fe3Pt, FePt, and FePt3 using differential scanning calorimetry. J. Appl. Phys. 110, 013903 (2011)
Chen, M., Liu, J.P., Sun, S.: One-step synthesis of FePt nanoparticles with tunable size. J. Am. Chem. Soc. 126(27), 8394–8395 (2004)
Iwamoto, T., Matsumoto, K., Kitamoto, Y., Toshima, N.: Direct synthesis of fct-structured FePt nanoparticles at low temperature with assistance of poly(N-vinyl-2-pyrrolidone). J. Colloid Interface Sci. 308(2), 564–567 (2007)
Iwamoto, T., Matsumoto, K., Matsushita, T., Inokuchi, M., Toshima, N.: Direct synthesis and characterizations of fct-structured FePt nanoparticles using poly(N-vinyl-2-pyrrolidone) as a protecting agent. J. Colloid Interface Sci. 336(2), 879–888 (2009)
Liu, F., Zhu, J.H., Yang, W.L., Dong, Y.H., Hou, Y.L., Zhang, C.Z., Yin, H., Sun, S.H.: Building nanocomposite magnets by coating a hard magnetic core with a soft magnetic shell. Angew. Chem.-Int. Edit. 53, 2176–2180 (2014)
Sun, S., Murray, C.B., Weller, D., Folks, L., Moser, A.: Monodisperse FePt nanoparticles and ferromagnetic FePt nanocrystal superlattices. Science. 287, 1989–1992 (2000)
Yu, J., Gao, W., Liu, F., Ju, Y., Zhao, F., Yang, Z., et al.: Tuning crystal structure and magnetic property of dispersible FePt intermetallic nanoparticles. Sci. China Mater. 61(7), 961–968 (2018)
Shinoda, K., Sato, K., Jeyadevan, B., Tohji, K., Suzuki, S.: Local structural studies of directly synthesized L10 FePt nanoparticles by using XRD, XAS and ASAXS. J. Magn. Magn. Mater. 310(2), 2387–2389 (2007)
Wierman, K.W., Platt, C.L., Howard, J.K.: Impact of stoichiometry on L10 ordering in FePt and FePtCu thin films. J. Magn. Magn. Mater. 278(1–2), 214–217 (2004)
Balachandran, J., Kiyoshi, U., Akira, H., Nallasamy, C., Kozo, S., Kazuyuki, T., et al.: Direct synthesis of fct-FePt nanoparticles by chemical route. Jpn. J. Appl. Phys. 42(4A), L350 (2003)
Acknowledgements
This work was supported by the National Nature Science Foundation of China through Grant No. U1809215 and the Zhejiang Provincial Natural Science Foundation of China through Grant No. LY18E020016.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Yu, J., Han, D., Ying, Y. et al. Influence of Particle Size on the Spin Pinning Effect in the fcc-FePt Nanoparticles. J Supercond Nov Magn 32, 1501–1505 (2019). https://doi.org/10.1007/s10948-019-5091-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10948-019-5091-7