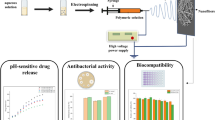
Abstract
Timely recognition the on-set of infection is very important for management and treatment of wounds. Regarding various biomarkers, pH of the wound milieu could be mentioned as one of the most effective tools for this purpose. In this work, some smart dressings were fabricated for detection of the change of pH in wounds due to infection. To do this, phenol red as a pH-indicator dye was incorporated into an alginate-based matrix. Two groups of dressings containing phenol red were prepared via freeze-drying and electrospinning techniques. Pore size of the freeze-dried dressings as well as mean diameter and pores size of the electrospun samples were estimated to be 62.24 μm, 204 nm, and 2.03 μm, respectively. Remarkable fluid absorption was determined for both electrospun (2125%) and freeze-dried (860%) samples. Measurements according to CIE L*a*b* and RGB color spaces revealed that the color difference between samples in buffers with different pHs of 4, 6, 7, 8, 9, and 10 could be distinguished well by the naked eye. In spite of less fluid absorption for the freeze-dried samples in comparison to electrospun ones, they exhibited better resistance to dye leaching and preserved their structure better after soaking in different pH buffers. Biological evaluation using adipose-derived mesenchymal stem cells, revealed no cytotoxic effects for samples containing phenol red. Moreover, the alginate matrix provides proper conditions for attachment as well as proliferation of the cells. Overall, the results suggest that samples which were fabricated through freeze-drying method could be considered as good candidates for application in management and monitoring of chronic wounds.





Similar content being viewed by others
References
S. Ayu Lestari, M. Armayah, Modern Commercial Wound Dressings and Introducing New Wound Dressings for Wound Healing: A Revie, Polymerization. 8 (2016) 65–80
A.R. Sadeghi-Avalshahr, S. Nokhasteh, A.M. Molavi, N. Mohammad-Pour, M. Sadeghi, Tailored PCL scaffolds as skin substitutes using sacrificial PVP fibers and collagen/chitosan blends. Int. J. Mol. Sci. 21 (2020). https://doi.org/10.3390/ijms21072311
Functional wound dressings.pdf, (n.d.)
L. Liu, X. Li, M. Nagao, A.L. Elias, R. Narain, H.J. Chung, A pH-Indicating colorimetric tough hydrogel patch towards applications in a substrate for smart wound dressings, polymers (Basel). 9 (2017). https://doi.org/10.3390/polym9110558
Color changing of, smart fibrous materials for naked eye real-time monitoring of wound pH.pdf, (n.d.)
M.B. Bazbouz, G. Tronci, Two-layer electrospun system enabling wound exudate management and visual infection response. Sens. (Switzerland). 19 (2019). https://doi.org/10.3390/s19050991
J. Chalitangkoon, P. Monvisade, Synthesis of chitosan-based polymeric dyes as colorimetric pH-sensing materials: potential for food and biomedical applications. Carbohydr. Polym. 260 (2021). https://doi.org/10.1016/j.carbpol.2021.117836
A.A. Arafa, A.A. Nada, A.Y. Ibrahim, P. Sajkiewicz, M.K. Zahran, O.A. Hakeim, Preparation and characterization of smart therapeutic pH-sensitive wound dressing from red cabbage extract and chitosan hydrogel. Int. J. Biol. Macromol. 182, 1820–1831 (2021). https://doi.org/10.1016/j.ijbiomac.2021.05.167
P. Kassal, M. Zubak, G. Scheipl, G.J. Mohr, M.D. Steinberg, I. Murković, Steinberg, Smart bandage with wireless connectivity for optical monitoring of pH. Sens. Actuators B Chem. 246, 455–460 (2017). https://doi.org/10.1016/j.snb.2017.02.095
C. Gamerith, D. Luschnig, A. Ortner, N. Pietrzik, J.H. Guse, M. Burnet, M. Haalboom, J. van der Palen, A. Heinzle, E. Sigl, G.M. Gübitz, pH-responsive materials for optical monitoring of wound status. Sens. Actuators B Chem. 301 (2019). https://doi.org/10.1016/j.snb.2019.126966
A. Truskewycz, V.K. Truong, A.S. Ball, S. Houshyar, N. Nassar, H. Yin, B.J. Murdoch, I. Cole, Fluorescent Magnesium Hydroxide Nanosheet Bandages with tailored Properties for Biocompatible Antimicrobial Wound Dressings and pH monitoring. ACS Appl. Mater. Interfaces. 13, 27904–27919 (2021). https://doi.org/10.1021/acsami.1c05908
S. Li, H. Vu, J. Senkowsky, W. Hu, L. Tang, A near-infrared fluorescent pH sensing film for wound milieu pH monitoring. Exp. Dermatol. 29, 107–111 (2020). https://doi.org/10.1111/exd.14046
A. Agarwal, A. Raheja, T.S. Natarajan, T.S. Chandra, Sensors and actuators B: Chemical Development of universal pH sensing electrospun nanofibers. Sens. Actuators B Chem. 161, 1097–1101 (2012)
alginate 2021.pdf, (n.d.)
G. Schoukens, Bioactive dressings to promote wound healing. Adv. Text. Wound Care. 135–167 (2019). https://doi.org/10.1016/b978-0-08-102192-7.00005-9
K.Y. Lee, D.J. Mooney, Alginate: Properties and biomedical applications. Prog Polym. Sci. 37, 106–126 (2012). https://doi.org/10.1016/j.progpolymsci.2011.06.003
S.G. Reddy, A.-A.S. Product, Its Properties and Applications, Prop. Appl. Alginates. (2021). https://doi.org/10.5772/INTECHOPEN.98831
Perspectives of chitosan, and alginate membranes for biomedical applications | Request PDF, (n.d.)
M.I. Neves, L. Moroni, C.C. Barrias, Modulating Alginate Hydrogels for Improved Biological Performance as Cellular 3D microenvironments, Front. Bioeng. Biotechnol. 8, 665 (2020). https://doi.org/10.3389/FBIOE.2020.00665/BIBTEX
Alginate, in Wound Dressings.pdf, (n.d.)
S. Dhivya, V.V. Padma, E. Santhini, Wound dressings – a review.pdf, (2015) 24–28
Z.M. Huang, Y.Z. Zhang, M. Kotaki, S. Ramakrishna, A review on polymer nanofibers by electrospinning and their applications in nanocomposites. Compos. Sci. Technol. 63, 2223–2253 (2003). https://doi.org/10.1016/S0266-3538(03)00178-7
M.S. Islam, B.C. Ang, A. Andriyana, A.M. Afifi, A review on fabrication of nanofibers via electrospinning and their applications. SN Appl. Sci. 1 (2019). https://doi.org/10.1007/s42452-019-1288-4
N. Bhattarai, D. Edmondson, O. Veiseh, F.A. Matsen, M. Zhang, Electrospun chitosan-based nanofibers and their cellular compatibility. Biomaterials. 26, 6176–6184 (2005). https://doi.org/10.1016/j.biomaterials.2005.03.027
Chap, 10 - Centrifugal Spinning—High Rate Production of Nanofibers.pdf, n.d
L.H. Chong, N.Z. Zarith, N. Sultana, Poly(Caprolactone)/chitosan-based scaffold using freeze drying technique for bone tissue engineering application, 2015 10th Asian Control Conf. Emerg. Control Tech. a Sustain. World, ASCC 2015. (2015). https://doi.org/10.1109/ASCC.2015.7244570
Shukla, FREEZE DRYING PROCESS: A REVIEW Soham Shukla* Department of Pharmaceutical Technology, Patel Pharmacy College, Saffrony Institute of Technology, Near Saffrony Holiday Resort, Ahmedabad-Mehsana Highway, at & Post Linch-384435, Mahesana, Gujarat, Indi. Int. J. Pharm. Sci. Res. 2, 3061–3068 (2011). https://ijpsr.com/bft-article/freeze-drying-process-a-review/?view=fulltext
C.A. Bonino, M.D. Krebs, C.D. Saquing, S.I. Jeong, K.L. Shearer, E. Alsberg, S.A. Khan, Electrospinning alginate-based nanofibers: from blends to crosslinked low molecular weight alginate-only systems. Carbohydr. Polym. 85, 111–119 (2011). https://doi.org/10.1016/j.carbpol.2011.02.002
K. Tarun, N. Gobi, Calcium alginate/PVA blended nano fibre matrix for wound dressing. Indian J. Fibre Text. Res. 37, 127–132 (2012)
S. Alborzi, L.T. Lim, Y. Kakuda, Electrospinning of sodium alginate-pectin ultrafine fibers. J. Food Sci. 75, 100–107 (2010). https://doi.org/10.1111/j.1750-3841.2009.01437.x
H. Hajiali, J.A. Heredia-Guerrero, I. Liakos, A. Athanassiou, E. Mele, Alginate Nanofibrous Mats with adjustable degradation rate for Regenerative Medicine. Biomacromolecules. 16, 936–943 (2015). https://doi.org/10.1021/bm501834m
M. Castellano, M. Alloisio, R. Darawish, A. Dodero, S. Vicini, Electrospun composite mats of alginate with embedded silver nanoparticles: synthesis and characterization. J. Therm. Anal. Calorim. 137, 767–778 (2019). https://doi.org/10.1007/s10973-018-7979-z
N. Bhattarai, M. Zhang, Controlled synthesis and structural stability of alginate-based nanofibers. Nanotechnology. 18 (2007). https://doi.org/10.1088/0957-4484/18/45/455601
BS EN, 13726-1-2002.pdf, (n.d.)
CIE_Lab_info.pdf, (n.d.)
H. Wen, W. Xiao, S. Biswas, Z.Q. Cong, X.M. Liu, K.S. Lam, Y.H. Liao, W. Deng, Alginate Hydrogel modified with a ligand interacting with α3β1 integrin receptor promotes the differentiation of 3D neural Spheroids toward Oligodendrocytes in Vitro. ACS Appl. Mater. Interfaces. 11, 5821–5833 (2019). https://doi.org/10.1021/acsami.8b19438
N. Bahrami, A. Farzin, F. Bayat, A. Goodarzi, M. Salehi, R. Karimi, A. Mohamadnia, A. Parhiz, J. Ai, Optimization of 3D Alginate Scaffold Properties with interconnected porosity using freeze-drying method for cartilage tissue Engineering Application. Arch. Neurosci. 6, 4–11 (2019). https://doi.org/10.5812/ans.85122
A. Sergeeva, N. Feoktistova, V. Prokopovic, D. Gorin, D. Volodkin, Design of porous Alginate Hydrogels by Sacrificial CaCO3 templates: pore formation mechanism. Adv. Mater. Interfaces. 2, 1–10 (2015). https://doi.org/10.1002/admi.201500386
A. Vincent, Study of Porosity of Gelatin-Alginate Hydrogels to Model Brain Study of Porosity of Gelatin-Alginate Hydrogels to Model Brain Matter for Studying Traumatic Brain Injuries Matter for Studying Traumatic Brain Injuries, (2022). https://opencommons.uconn.edu/srhonors_theses/887
V.A. Petrova, A.S. Golovkin, A.I. Mishanin, D.P. Romanov, D.D. Chernyakov, D.N. Poshina, Y.A. Skorik, Cytocompatibility of Bilayer Sca ff olds Electrospun from Nanowhiskers, Chitosan Alginate-chitin. Biomedicines. 8, 305 (2020)
B. Vigani, S. Rossi, G. Sandri, M.C. Bonferoni, G. Milanesi, G. Bruni, F. Ferrari, Coated electrospun alginate-containing fibers as novel delivery systems for regenerative purposes. Int. J. Nanomedicine. 13, 6531–6550 (2018). https://doi.org/10.2147/IJN.S175069
R. Wongkanya, P. Chuysinuan, C. Pengsuk, S. Techasakul, K. Lirdprapamongkol, J. Svasti, P. Nooeaid, Electrospinning of alginate/soy protein isolated nanofibers and their release characteristics for biomedical applications. J. Sci. Adv. Mater. Devices. 2, 309–316 (2017). https://doi.org/10.1016/j.jsamd.2017.05.010
E. U.M.D.E.C.D, Los, No 主観的健康感を中心とした在宅高齢者における 健康関連指標に関する共分散構造分析 Title, (n.d.).
J. Grenier, H. Duval, F. Barou, P. Lv, B. David, D. Letourneur, Mechanisms of pore formation in hydrogel scaffolds textured by freeze-drying. Acta Biomater. 94, 195–203 (2019). https://doi.org/10.1016/j.actbio.2019.05.070
M. Guastaferro, L. Baldino, E. Reverchon, S. Cardea, Production of porous agarose-based structures: freeze-drying vs. supercritical CO2 drying. Gels. 7 (2021). https://doi.org/10.3390/gels7040198
Z. Zhang, Y. Feng, L. Wang, D. Liu, C. Qin, Y. Shi, A review of preparation methods of porous skin tissue engineering scaffolds. Mater. Today Commun. 32, 104109 (2022). https://doi.org/10.1016/J.MTCOMM.2022.104109
Y. Zhang, M. Zhang, D. Cheng, S. Xu, C. Du, L. Xie, W. Zhao, Applications of electrospun scaffolds with enlarged pores in tissue engineering. Biomater. Sci. 10, 1423–1447 (2022). https://doi.org/10.1039/D1BM01651B
I. Negut, G. Dorcioman, V. Grumezescu, Scaffolds for Wound Healing Applications, Polymers (Basel). 12 (2020) 1–19. https://doi.org/10.3390/POLYM12092010
A. Deng, Y. Yang, S. Du, Tissue Engineering 3D Porous Scaffolds Prepared from Electrospun Recombinant Human Collagen (RHC) Polypeptides/Chitosan Nanofibers, Appl. Sci. 2021, Vol. 11, Page 5096. 11 (2021) 5096. https://doi.org/10.3390/APP11115096
A. Ahmed, G. Getti, J. Boateng, Ciprofloxacin-loaded calcium alginate wafers prepared by freeze-drying technique for potential healing of chronic diabetic foot ulcers. Drug Deliv. Transl. Res. 8, 1751–1768 (2018). https://doi.org/10.1007/s13346-017-0445-9
T.C. Trevisol, A.R.M. Fritz, S.M.A.G.U. de Souza, A.C.K. Bierhalz, J.A.B. Valle, Alginate and carboxymethyl cellulose in monolayer and bilayer films as wound dressings: Effect of the polymer ratio. J. Appl. Polym. Sci. 136 (2019). https://doi.org/10.1002/app.46941
K.R. Aadil, A. Nathani, C.S. Sharma, N. Lenka, P. Gupta, Fabrication of biocompatible alginate-poly(vinyl alcohol) nanofibers scaffolds for tissue engineering applications. Mater. Technol. 33, 507–512 (2018). https://doi.org/10.1080/10667857.2018.1473234
T.K.V. Urquiza, O.P. Pérez, M.G. Saldaña, Effect of the cross-linking with calcium ions on the structural and thermo-mechanical properties of alginate films, Mater. Res. Soc. Symp. Proc. 1355 (2011) 16–21. https://doi.org/10.1557/opl.2011.1136
S.Y. Park, W.J. Kim, J.B. Choi, S. Kim, Physical and mechanical properties of alginate-based hydrogel film as carrier for release of acetylthiocholine. Int. J. Precis Eng. Manuf. 19, 129–135 (2018). https://doi.org/10.1007/s12541-018-0015-1
T.M.S. Udenni Gunathilake, Y.C. Ching, K.Y. Ching, C.H. Chuah, L.C. Abdullah, Biomedical and microbiological applications of bio-based porous materials: a review. Polym. (Basel). 9, 1–16 (2017). https://doi.org/10.3390/polym9050160
D. Wu, F. Xu, B. Sun, R. Fu, H. He, K. Matyjaszewski, Design and preparation of porous polymers. Chem. Rev. 112, 3959–4015 (2012). https://doi.org/10.1021/CR200440Z. /ASSET/IMAGES/CR200440Z.SOCIAL.JPEG_V03
L. De Smet, G. Vancoillie, P. Minshall, K. Lava, I. Steyaert, E. Schoolaert, E. Van De Walle, P. Dubruel, K. De Clerck, R. Hoogenboom, Plasma dye coating as straightforward and widely applicable procedure for dye immobilization on polymeric materials. Nat. Commun. 2018. 91(9), 1–11 (2018). https://doi.org/10.1038/s41467-018-03583-4
T. De Meyer, I. Steyaert, K. Hemelsoet, R. Hoogenboom, V. Van Speybroeck, K. De Clerck, Halochromic properties of sulfonphthaleine dyes in a textile environment: the influence of substituents. Dye. Pigment. 124, 249–257 (2016). https://doi.org/10.1016/J.DYEPIG.2015.09.007
E. Schoolaert, I. Steyaert, G. Vancoillie, J. Geltmeyer, K. Lava, R. Hoogenboom, K. De Clerck, Blend electrospinning of dye-functionalized chitosan and poly(ε-caprolactone): towards biocompatible pH-sensors. J. Mater. Chem. B 4, 4507–4516 (2016). https://doi.org/10.1039/C6TB00639F
M. Ernst, C. Schmid, E.R. Froesch, Phenol red mimics biological actions of estradiol: enhancement of osteoblast proliferation in vitro and of type I collagen gene expression in bone and uterus of rats in vivo. J. Steroid Biochem. 33, 907–914 (1989). https://doi.org/10.1016/0022-4731(89)90239-2
A. Morgan, D. Babu, B. Reiz, R. Whittal, L.Y.K. Suh, A.G. Siraki, Caution for the routine use of phenol red - it is more than just a pH indicator. Chem. Biol. Interact. 310 (2019). https://doi.org/10.1016/J.CBI.2019.108739
N. Kuncharoenwirat, W. Chatuphonprasert, K. Jarukamjorn, Effect of phenol red on cell cultures. Isan J. Pharm. Sci. IJPS (Isan J. Pharm. Sci). 17, 13–23 (2021). https://doi.org/10.14456/IJPS.2021.2
R. Phenol, powder, BioReagent, cell culture mammalian 143-74-8, (n.d.)
N. Ansari, S. Müller, E.H.K. Stelzer, F. Pampaloni, Quantitative 3D cell-based assay performed with Cellular Spheroids and fluorescence Microscopy. Methods Cell. Biol. 113, 295–309 (2013). https://doi.org/10.1016/B978-0-12-407239-8.00013-6
P. Held, D. Ph, B. Instruments, p p l i c a t i o n o t e Using Phenol Red to Assess pH in Tissue Culture Media, (1966)
L. Bennison, C. Miller, R. Summers, A. Minnis, G. Sussman, W. McGuiness, Bennison, 25 (2017) 63–69
E.M. Jones, C.A. Cochrane, S.L. Percival, The Effect of pH on the Extracellular Matrix and Biofilms. Adv. Wound Care. 4, 431–439 (2015). https://doi.org/10.1089/wound.2014.0538
S.L. Percival, S. McCarty, J.A. Hunt, E.J. Woods, The effects of pH on wound healing, biofilms, and antimicrobial efficacy. Wound Repair. Regen. 22, 174–186 (2014). https://doi.org/10.1111/wrr.12125
Y. Hwang, N. Sangaj, S. Varghese, Interconnected macroporous poly(Ethylene Glycol) cryogels as a cell scaffold for cartilage tissue engineering. Tissue Eng. - Part. A 16, 3033–3041 (2010). https://doi.org/10.1089/ten.tea.2010.0045
Author information
Authors and Affiliations
Contributions
This manuscript is result of a joint work of authors and all of them have participated in this work and they have approved its final version for publication. All authors interpreted the data and discussed the results throughout the project duration.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest for this research paper.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Haghbin, M., Sadeghi-Avalshahr, A., Hassanzadeh, H. et al. Preparation of porous alginate-based smart dressings used in real-time monitoring of pH in chronic wounds by evaluating two fabrication routes: freeze-drying vs. electrospinning. J Porous Mater 30, 1953–1963 (2023). https://doi.org/10.1007/s10934-023-01477-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10934-023-01477-5