Abstract
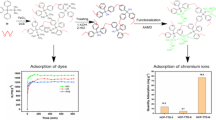
Aimed to prepare high efficient dye sorbent and control water pollution, herein we utilized solvothermal method to synthesize porous polyimide (PI) polymer with a large surface area using DMSO as solvent. Unlike the solid-state thermal polymerized PI with low surface area of 5 m2g−1, this PI material prepared in DMSO solvent possessed a large surface area of 430 m2g−1, which was beneficial for adsorption of organic dye in waste water, achieving a max MO adsorption of 200 mg g−1 three times higher than that of multiwalled carbon nanotube. The adsorption kinetics of dye molecules on PI was investigated in detail and the R2 value of 0.99071 for pseudo-second-order model confirms the adsorption was fitted best with Langmuir isotherm.









Similar content being viewed by others
References
S.H. Chen, J. Zhang, C.L. Zhang, Q.Y. Yue, Y. Li, C. Li, Equilibrium and kinetic studies of methyl orange and methyl violet adsorption on activated carbon derived from Phragmites australis. Desalination. 252, 149–156 (2010)
A.E. Ofomaja, Y.S. Ho, Effect of temperatures and pH on methyl violet biosorption by Mansonia wood sawdust. Bioresour. Technol. 99, 5411–5417 (2008)
Z. Aksu, Application of biosorption for the removal of organic pollutants: a review. Process Biochem. 40, 997–1026 (2005)
G. McKay, Adsorption of dyestuffs from aqueous solutions with activated carbon. Part I. Equilibrium and batch contact-time studies. J. Chem. Technol. Biotechnol. 32, 759–772 (1982)
P. Nigam, I.M. Banat, D. Singh, R. Marchant, Microbial process for decolorization of textile effluent containing azo, diazo and reactive dyes. Process Biochem. 31 (1996) 435–442
G. Annadurai, R.-S. Juang, D.-J. Lee, Use of cellulose-based wastes for adsorption of dyes from aqueous solutions. J. Hazard. Mater. 92, 263–274 (2002)
Y.L. Yao, B. He, F.F. Xu, X.F. Chen, Equilibrium and kinetic studies of methyl orange adsorption on multiwalled carbon nanotubes. Chem. Eng. J. 170, 82–89 (2011)
Z.M. Ni, S.J. Xia, L.G. Wang, F.F. Xing, G.X. Pan, Treatment of methyl orange by calcined layered double hydroxides in aqueous solution: adsorption property and kinetic studies. J. Colloid Interface Sci. 316, 284–291 (2007)
M. Küçükosmanoğlu, O. Gezici, A. Ayar, The adsorption behaviors of methylene blue and methyl orange in a diaminoethane sporopollenin-mediated column system. Sep. Purif. Technol. 52, 280–287 (2006)
J.H. Huang, K.L. Huang, S.Q. Liu, A.T. Wang, C. Yan, Adsorption of rhodamine B and methyl orange on a hypercrosslinked polymeric adsorbent in aqueous solution. Colloids Surf. A 330, 55–61 (2008)
M. Samarghandi, M. Hadi, S. Moayedi, F. Askari, Two-parameter isotherms of methyl orange sorption by pinecone derived activated carbon. Iran. J. Environ. Health Sci. Eng. 6, 285–294 (2009)
Z.N. Liu, A.N. Zhou, G.R. Wang, X.G. Zhao, Adsorption behavior of methyl orange onto modified ultrafine coal powder. Chin. J. Chem. Eng. 17, 942–948 (2009)
S. Chu, Y. Wang, Y. Guo, P. Zhou, H. Yu, L.L. Luo, F. Kong, Z.G. Zou, Facile green synthesis of crystalline polyimide photocatalyst for hydrogen generation from water. J. Mater. Chem. 22, 15519–15521 (2012)
M.S. Ahola, E.S. Sailynoja, M.H. Raitavuo, Invitro release of heparin from silica xerogels. Biomaterials. 22, 2163–2170 (2001)
S. Park, K. Li, F. Jin, Synthesis and characterization of hyper-branched polyimides from 2,4,6-triamino pyrimidine and dianhydrides system. Mater. Chem. Phys. 108, 214–219 (2008)
S. Chu, Y. Wang, C. Wang, J. Yang, Z. Zou, Bandgap modulation of polyimide photocatalyst for optimum H2 production activity under visible light irradiation. Int. J. Hydrog. Energy. 38, 10768–10772 (2013)
S.Z. Zeng, L.M. Guo, F.M. Cui, Z. Gao, J. Zhou, J.L. Shi, In situ self-assembly of zigzag polyimide chains to crystalline branched supramolecular structures with high surface area. Macromol. Chem. Phys. 211, 698–705 (2010)
S. Brauner, P.H. Emmet, E. Teller, Adsorption of gases in multimolecular layers. J. Am. Chem. Soc. 60, 309–319 (1938)
A. Trewin, A.I. Cooper, Predicting microporous crystalline polyimides. CrystEngComm. 11, 1819–1822 (2009)
S. Khan, J. Gor, B. Mulloy, S.J. Perkins, Semi-rigid solution structures of heparin by constrained X-ray scattering modelling: new insight into heparin-protein complexes. J. Mol. Biol. 395, 504–521 (2010)
M.M. Wan, W.J. Qian, W.G. Lin, Y. Zhou, J.H. Zhu, Multiply functionalization of SBA-15 mesoporous silica in one-pot: fabricating aluminum- containing plugged composite for heparin sustaining release. J. Mater. Chem. B. 1, 3897–3905 (2013)
K. Park, G.Y. Lee, Y.S. Kim, M. Yu, R.W. Park, I.S. Kim, S.Y. Kim, Y. Byun, Heparin-deoxycholic acid chemical conjugated as an anticancer drug carrier and its antitumor activity. J. Control. Release. 114, 300–306 (2006)
V. Hoffart, A. Lamprecht, P. Maincent, T. Lecompte, C. Vigneron, N. Ubrich, Oral bio-availability of a low molecular weight heparin using a polymeric delivery system. J. Controlled Release. 113, 38–42 (2006)
I.A.W. Tan, B.H. Hameed, A.L. Ahmad, Equilibrium and kinetic studies on basic dye adsorption by oil palm fibre activated carbon. Chem. Eng. J. 127, 111–119 (2007)
M. El-Guendi, Homogeneous surface diffusion model of basic dyes tuffs onto natural clay in batch adsorbers. Adsorpt. Sci. Technol. 8, 217–225 (1991)
H. Freundlich, Over the adsorption in solution. J. Phys. Chem. 57, 385–470 (1906)
F. Haghseresht, G. Lu, Adsorption characteristics of phenolic compounds onto coal-reject-derived adsorbents. Energy Fuels. 12, 1100–1107 (1998)
I. Langmuir, The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 40, 1361–1403 (1918)
K.R. Hall, L.C. Eagleton, A. Acrivos, T. Vermeulen, Pore- and solid-diffusion kinetics in fixed-bed adsorption under constant-pattern conditions. Ind. Enq. Chem. Fundam. 5, 212–223 (1966)
T.W. Weber, R.K. Chakkravorti, Pore and solid diffusion models for fixed-bed adsorbers. AlChE J. 20, 228–238 (1974)
S. Lagergren, About the theory of so-called adsorption of soluble substances. Kungliga Svenska Vetens kapsaka demiens Handlingar, 24(1898), 1–39
Y.S. Ho, G. McKay, Pseudo-second order model for sorption processes. Process Bio chem. 34, 451–465 (1999)
W.J. Weber Jr., J.C. Morris, Kinetics of adsorption on carbon from solution. J. Sanit. Eng. Div. Am. Soc. Civ. Eng. 89, 31–60 (1963)
M. Özacar, I.A. Şengil, Adsorption of reactive dyes on calcined alunite from aqueous solutions. J. Hazard. Mater. 40, 1–14 (2003)
Z. Aksu, G. Do onmez, A comparative study on the biosorption characteristics of some yeasts for Remazol Blue reactive dye. Chemosphere. 50, 1075–1083 (2003)
Z. Eren, F.N. Acar, Adsorption of reactive black 5 from an aqueous solution: equilibrium and kinetic studies. Desalination. 194, 1–10 (2006)
Acknowledgements
This work was financially supported by NSFC (21773113), and the authors would like to thank Analysis Center of Nanjing University for the sample characterization and the National Supercomputing Center in Shenzhen for computational facilities.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhu, H., Wang, C., Zhou, J. et al. Solvothermal synthesis of porous conjugated polymer with high surface area for efficient adsorption of organic and biomolecules. J Porous Mater 25, 1659–1668 (2018). https://doi.org/10.1007/s10934-018-0579-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10934-018-0579-2