Abstract
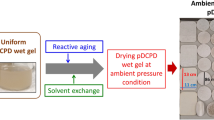
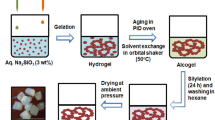
The aim of this paper is to synthesis a new type of insulation material (EPA), which fill the aerogels into the expanded perlite (EP). EP is a kind of lightweight filler, its application is constrained by the character of absorbing water easily. The silicic acid is inhaled into the expanded perlite at −0.1 MPa, aging 24 h, it becomes aerogel after solvent exchanging/surface modifying and drying. The pores of EP are filled with aerogel which affects thermal conductivity of expanded perlite little and makes it hydrophobic. EPA has a wider use than EP for its hydrophobic character. A new recipe to synthesize aerogel, filled into EP, with the thermal conductivity of 0.034 W/m K in ambient pressure drying is found in this experiment. The time and reagents dosage for synthesizing EPA are less than large block of aerogel, while the thermal conductivity is close to it. The scanning electron microscopy is used to analyze EPA’s micro structure. The thermal conductivity tester is used for testing the thermal conductivity of silica aerogel, expanded perlite and EPA.












Similar content being viewed by others
References
C.J. Lee, G.S. Kim, S.H. Hyun, Synthesis of silica aerogels from waterglass via new modified ambient drying. J. Mater. Sci. 37, 2237–2241 (2002)
G. Carlson, D.L.K. McKinley, J. Richardson, T. Tillotson, Aerogel commercialization technology, markets and costs. J. Non-Cryst. Solids 186, 372–379 (1995)
E.N. Mari-Ann Einarsrud, Strengthening of water glass and colloidal sol based silica gels by aging in TEOS. J. Non-Cryst. Solids 226, 7 (1998)
P.B. Sarawade et al., Influence of aging conditions on textural properties of water-glass-based silica aerogels prepared at ambient pressure. Korean J. Chem. Eng. 27(4), 1301–1309 (2010)
M.D.F. Júlio, L.M. Ilharco, Superhydrophobic hybrid aerogel powders from waterglass with distinctive applications. Microporous Mesoporous Mater. 199, 29–39 (2014)
F. Shi, L. Wang, J. Liu, Synthesis and characterization of silica aerogels by a novel fast ambient pressure drying process. Mater. Lett. 60, 3718–3722 (2006)
G. Wu et al., Preparation and surface modification mechanism of silica aerogels via ambient pressure drying. Mater. Chem. Phys. 129(1–2), 308–314 (2011)
A.P. Rao, A.V. Rao, U.K.H. Bangi, Low thermalconductive, transparent and hydrophobic ambient pressure dried silica aerogels with various preparation conditions using sodium silicate solutions. J. Sol-Gel Sci. Technol. 47(1), 85–94 (2008)
P.M. Shewale et al., Synthesis and characterization of low density and hydrophobic silica aerogels dried at ambient pressure using sodium silicate precursor. J. Porous Mater. 16(1), 101–108 (2007)
I.B. Topçu, B. Işıkdağ, Manufacture of high heat conductivity resistant clay bricks containing perlite. Build. Sci. 42(10), 3540–3546 (2007)
A.G. Celik, A.M. Kilic, G.O. Cakal, Expanded perlite aggregate characterization for use as a lightweight construction raw material. Physicochem. Probl. Miner. Process. 49(2), 689–700 (2013)
A. Sarı, A. Karaipekli, C. Alkan, Preparation, characterization and thermal properties of lauric acid/expanded perlite as novel form-stable composite phase change material. Chem. Eng. J. 155(3), 899–904 (2009)
R. Demirboǧa, R. Gül, Thermal conductivity and compressive strength of expanded perlite aggregate concrete with mineral admixtures. Energ. Buildings 35(11), 1155–1159 (2003)
O. Sengul et al., Effect of expanded perlite on the mechanical properties and thermal conductivity of lightweight concrete. Energ Build. 43(2–3), 671–676 (2011)
M. Alam et al., Experimental characterisation and evaluation of the thermo-physical properties of expanded perlite—fumed silica composite for effective vacuum insulation panel (VIP) core. Energ Build. 69, 442–450 (2014)
S. Ramakrishnan et al., A novel paraffin/expanded perlite composite phase change material for prevention of PCM leakage in cementitious composites. Appl. Energy 157, 85–94 (2015)
N. Zhang et al., Lauric–palmitic–stearic acid/expanded perlite composite as form-stable phase change material: Preparation and thermal properties. Energ Build. 82, 505–511 (2014)
J. Zhang et al., Preparation and properties of gypsum based energy storage materials with capric acid–palmitic acid/expanded perlite composite PCM. Energ Build. 92, 155–160 (2015)
A. Karaipekli et al., Thermal characteristics of expanded perlite/paraffin composite phase change material with enhanced thermal conductivity using carbon nanotubes. Energy Convers. Manag. 134, 373–381 (2017)
R. Li et al., Thermal compatibility of Sodium Nitrate/Expanded Perlite composite phase change materials. Appl. Therm. Eng. 103, 452–458 (2016)
A.P. Rao, A.V. Rao, G.M. Pajonk, P.M. Shewale, Effect of solvent exchanging process on the preparation of the hydrophobic silica aerogels by ambient pressure drying method using sodium silicate precursor. J. Mater. Sci. 42, 8418–8425 (2007)
Rao, A.P., A.V. Rao, G.M. Pajonk, Hydrophobic and physical properties of the two step processed ambient pressure dried silica aerogels with various exchanging solvents. J. Sol-gel Sci. Technol. 36(3), 285–292 (2005)
M-Y. Xie, K-T. Fang, Admissibility and minimaxity of the uniform design measure in nonparametric regression model. J. Stat. Plan. Inference, 83(1), 101–111 (2000). doi:10.1016/S0378-3758(99)00089-0
K.T. Fang, D.K. Lin, P. Winker, Y. Zhang, Uniform design: theory and application. Technometrics, 42, 237–248 (2000)
Wang, B. et al., Progress in drying technology for nanomaterials. Dry. Technol. 23(1–2), 7–32 (2005)
S. Rajeshkumar, G.M. Anilkumar, S. Ananthakumar, K.G.K. Warrier, Role of drying techniques on the development of porosity in silica gels. Dry. Technol. 5 59–63 (1998)
A.V. Rao, E. Nilsen, M.A. Einarsrud, Effect of precursors, methylation agents and solvents on the physicochemical properties of silica aerogels prepared by atmospheric pressure druing method. J. Non-cryst Solids 296, 165–171 (2001)
A.P. Rao, A.V. Rao, J.L. Gurav, Effect of protic solvents on the physical properties of the ambient pressure dried hydrophobic silica aerogels using sodium silicate precursor. J. Porous Mater. 15, 507–512 (2007)
U.K.H. Bangi, A. Venkateswara Rao, A. Parvathy Rao, A new route for preparation of sodium-silicate-based hydrophobic silica aerogels via ambient-pressure drying. Sci. Technol. Adv. Mater. 9(3), 035006 (2016)
L.W. Hrubesh, R.W. Pekala, Thermal properties of organic and inorganic aerogels. J. Mater. Res. 9, 731–738 (1994)
J. Fricke, T. Tillotson, Aerogels production, characterization, and applications. Thin Solid Films 297, 212–223 (1997)
M.A. Aegerter, N. Leventis, M.M. Koebel, Aerogels Handbook, ed. by M.A. Aegerter. Advances in Sol-Gel Derived Materials and Technologiesed (Springer, New York, 2011)
G. Wei, Y. Liu, X. Zhang, F. Yu, X. Du, Thermal conductivities study on silica aerogel and its composite insulation materials. Int. J. Heat Mass Transf. 54, 2355–2366 (2011)
X. Lu, R. Caps, J. Fricke, C.T. Alviso, R.W. Pekala, Correlation between structure and thermal conductivity of organic aerogels. J. Non-Cryst. Solids. 188, 226–234 (1995)
Acknowledgements
This research was financially supported by Shanxi Province Science and Technology Project in Taiyuan University of Technology (No. 20150313014-1) and Henan Province Science and Technology Project in Xinyang Normal University (No. 152102310361). We thank Shangtianti Yihe Resources Development Co., Ltd, Xinyang, China, for providing the expanded perlite materials.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, L., Li, Z., Jing, Q. et al. Synthesis of composite insulation materials—expanded perlite filled with silica aerogel. J Porous Mater 25, 373–382 (2018). https://doi.org/10.1007/s10934-017-0448-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10934-017-0448-4