Abstract
Hypereutrophic conditions in lake ecosystems are generally associated with nutrient inputs from surrounding terrestrial landscapes. However, some systems can receive primary nutrient inputs through hydrologic connections such as rivers or canals. Lake Carlton, Florida, USA is a small, shallow, polymictic lake that ends a hydrologically connected string of lacustrine systems with hypereutrophic lakes Beauclair and Apopka. Lake Beauclair and Lake Apopka were connected hydrologically when a system of canals was constructed beginning in 1893 CE. These lakes have maintained hypereutrophic conditions despite extensive management to reduce nutrient inputs. Here, we collected a sediment core from Lake Carlton to accomplish two primary research objectives: 1) reconstruct the nutrient input for Lake Carlton throughout the last ~ 150 years to conduct source assessment, and 2) link primary producer changes with management actions between lakes Apopka, Beauclair, and Carlton. Paleolimnological tools were applied to a 165-cm sediment core and analyzed for bulk density, organic matter content, nutrients (C, N, P), photosynthetic pigments, and total microcystins. Sediments were dated using 210Pb and results indicate that the core represents over 150 years of sediment accumulation. Sedimentary nutrient concentrations show that the primary driver of nutrient inputs resulted from canal construction, beginning in 1893 CE, which corresponded to increased nutrient deposition. Photosynthetic pigment data indicate dramatic increases in most primary producer groups coinciding with the hydrologic modification. However, around ~ 1970 CE, primary producer communities shifted from diatom dominance to cyanobacterial dominance, which appeared to be linked to internal nutrient dynamics and competition among phytoplankters within the lake ecosystem. Cyanotoxin production records show a significant lag between cyanobacterial dominance and peak cyanotoxin production with toxins increasing in the last 30 years. These data demonstrate that local nutrient inputs do not govern all phytoplankton dynamics in shallow lake systems but must be interpreted considering hydrologic alterations and management practices.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Over the last 150 years, anthropogenic modifications have accelerated nutrient loading to aquatic systems, transformed terrestrial landscapes, and altered the global climate (Steffen et al. 2007). For lake ecosystems the acceleration of nutrient loading from agricultural activities and urbanization has increased net primary production and hypereutrophic conditions (Schindler 1977). Increased surface-water temperatures caused by global climate change can strengthen thermal stratification and lengthen optimal growing periods for phytoplankton as well as favor cyanobacteria growth and dominance (Paerl and Huisman 2008). Furthermore, the construction of canals and the modification of water levels can cause hydrologic alterations lowering lake levels and increasing nutrient and sediment loading (Waters et al. 2008). Intensified land use, sewage discharge, and climate change during the past two centuries further exacerbates the growth and proliferation of harmful algae in freshwaters favoring cyanobacteria species (Ho et al. 2019).
As lakes experience increased eutrophication due to cultural impacts on watersheds and lake ecosystems, cyanobacteria are given a competitive advantage over other phytoplankters (Heisler et al. 2008). Cyanobacteria are less nutritious for zooplanktonic predators, which can cause a negative bottom-up effect on higher trophic levels (Fulton and Paerl 1987). Fish and invertebrates lose habitat through a reduction in the number and diversity of aquatic plants and the lack of nutritional value from cyanobacteria (Fulton and Paerl 1987). This increase in cyanobacteria abundance can confound ecosystem services through anoxia, biological stress, and the production of toxins which can be hazardous to human health (Paerl and Huisman 2009). Though terrestrial nutrients are considered the primary drivers of phytoplankton dynamics in most lakes, additional stressors such as hydrologic change, internal nutrient loading, temperature increases, and fish management have also been shown to increase cyanobacteria abundance (Scheffer and van Ness 2007). As lakes begin to change due to these stressors, understanding how multiple drivers may impact primary producer community structure and ecosystem function are key for the predictability of lake change and for managers to make informed decisions.
Although nutrient inputs from terrestrial landscapes have been the focus of most lake eutrophication studies, recent studies have shown that hydrology can also be a driver of eutrophication and primary producer increase. Finlay et al. (2013) showed that hydrologic residence time related to primary producer growth and nitrogen (N) dynamics for a multi-lake dataset focused primarily on northern temperate lake ecosystems. Webster et al. (2021) showed that reservoirs connected in sequence can influence nutrient dynamics differently with phosphorus (P) related to the placement of the aquatic system in the reservoir sequence and N related to hydrologic residence time. Further research has shown that hydrologic alterations and connection through canals can cause cyanobacteria dominance both through decreases in water level contributing to changes in land-use practices (Waters et al. 2008) and pulses of nutrient-rich water (Waters et al. 2015). Whereas total nutrient inputs are of importance, these studies collectively demonstrate that hydrology can impact nutrient dynamics and biogeochemical processes post-input and subsequently impact primary producer communities.
Here, we collected a sediment core from Lake Carlton, Florida, USA to investigate the impacts of nutrient inputs, hydrologic alterations, and other stressors on primary producer community structure. Lake Carlton is connected to lakes Beauclair and Apopka, which form a string of lakes with a distinct nutrient source in Lake Apopka and known dates for hydrologic connectivity making Lake Carlton an ideal experimental system. The sediment core was analyzed for bulk density, organic matter, nutrients (C, N, P), photosynthetic pigments, and the cyanotoxin, microcystin. The primary research objectives were: 1) reconstruct the nutrient input and storage for Lake Carlton throughout the last 150 years to see if nutrient inputs result from hydrologic or local terrestrial inputs, and 2) compare primary producer change in response to management actions between lakes Apopka, Beauclair, and Carlton. We hypothesize that the primary nutrient source for Lake Carlton was Lake Apopka, which caused cyanobacterial dominance through time.
Methods
Study site
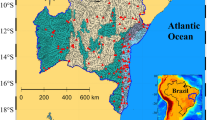
The Harris Chain of lakes in central Florida consists of eight primary lakes including lakes Apopka, Beauclair, Carlton, Dora, Eustis, Griffin, Harris, and Yale. This study focuses on three lakes in the southernmost portion of the Harris Chain (Fig. 1). These lakes were subject to large increases in anthropogenic nutrient loading and hydrologic modification beginning in 1893 CE. After a series of canals strengthened hydrologic connectivity between the lakes, poor agricultural practices around Lake Apopka led to widespread nutrient pollution throughout the Harris Chain (Schelske et al. 2005). Increased nutrient loading in Lake Apopka had downstream effects causing increased P concentrations and organic matter deposition in Lake Beauclair (Schelske et al. 2005). Due to the direct hydrologic connection to Lake Beauclair via the “Carlton Cut”, Lake Carlton went from an isolated lake to a connected member of the Harris Chain system. As both groundwater and surface water generally flow north in this region, the Florida Department of Environmental Protection (DEP) has theorized that P-rich surface waters are pushed from Lake Beauclair to Lake Carlton by wind-driven water movement and subsequent seiche (Amec 2018). Previous work used sediment-core analysis to determine the downstream effects Lake Apopka has had on other members of the Harris Chain of Lakes (Schelske et al. 2005), but the impacts to Lake Carlton were not considered. The methods used in Schelske et al. (2005) could be applied to sediment records obtained from Lake Carlton to extrapolate nutrient-pollution sources and compare eutrophication histories to a lake that has been altered from an isolated to a connected state.
Lake Carlton is a 1.53 km2 public lake situated between Orange County and Lake County, Florida, USA (°N 28.76020, °W 81.65741) (Fig. 1). Lake Carlton is connected to Lake Beauclair via the Carlton Cut but is not directly connected to any other lakes in the Harris Chain. Listed as “impaired” by the Florida DEP in 2002, Lake Carlton is a hypereutrophic, solution-depression lake currently dominated by cyanobacteria with Trophic Status Index (TSI) values ranging from 46 to 94, phosphorus concentrations ranging from 6 µg to 760 µg L−1, and chlorophyll-a (chl-a) concentrations ranging from 0.6 to 302 µg L−1. TSI values, nutrient concentrations, and pigment concentrations have decreased in recent years (Amec 2018). These large fluctuations in nutrient and algal concentrations are likely due to wind-driven pulses of nutrient-rich water from Lake Beauclair (Amec 2018).
Sample collection
Sediments from hypereutrophic systems in Florida are subject to wind-driven resuspension (Bachmann et al. 2000), which can lead to uneven sediment distribution within the basin (Whitmore et al. 1996). Based on soft sediment surveys from previous visits to Lake Carlton and bathymetric data, areas likely to facilitate sediment accumulation were chosen for the coring location. The core was collected at N 28.76170°, W 81.65514° at a water depth of 3.6 m, and a soft sediment depth of ~ 5 m. A sediment core was collected during the summer of 2020 using a piston-coring device that maintains an undisturbed sediment–water interface (Fisher et al. 1992). The core was collected in two sections with each section sampled in the field at 4-cm intervals. Sediment samples were stored on ice and in the dark until they were returned to the lab at Auburn University. Sectioning at 4-cm intervals, as opposed to smaller sections, was based on previous paleolimnological investigations in Florida (Brenner et al. 1999; Schelske et al. 2005; Waters et al. 2005) and to allow for enough sediment material for analytical measurements. A total of 42 intervals were collected and analyzed.
Laboratory analysis
An aliquot of known volume was removed from each sample for bulk density and gravimetric analysis. Bulk density was determined by weighing a known volume of wet sediment and re-weighing after drying in a drying oven. Bulk density is reported as g dry cm−3 wet. Organic matter content and non-volatile solids were determined through loss on ignition (LOI) at 550 °C in a muffle furnace for 3 h and reported as percent (Hakanson and Jansson 1983). Samples were then frozen, freeze-dried, and ground with a mortar and pestle for further analysis. Carbon and nitrogen were analyzed using a Costech Combustion Elemental Analyzer with an attached autosampler. For organic carbon, samples were acidified in HCl vapor for 24 h prior to analysis. For additional elements (P, Fe, S and other elements), sediment samples were analyzed using ICP-ARL following acid digestion in a heated block following EPA 6010B. This method is available from the Unites States Environmental Protection Agency website at https://www.epa.gov/sites/default/files/documents/6010b.pdf.
Fossilized photosynthetic pigments were measured using a high-performance liquid chromatography system (HPLC) following the methods of Leavitt and Hodgson (2001) and Waters (2016). Freeze-dried sediment samples were extracted with a solvent mixture of acetone, methanol, and water (80:15:5), which contains an internal standard (Sudan II: Sigma Chemical Corp.). Extraction was done for 16–24 h in a − 20 °C freezer. Samples were injected into the Shimadzu HPLC system following the mobile phase and time sequence of Leavitt and Hodgson (2001). Chlorophylls and carotenoids were separated by passing through a Phenomenex Luna C18 column and measured using a photodiode array detector coupled with a fluorescence detector. Pigments were identified using retention times and pigment-specific spectra of known standards. To compare chlorophylls and carotenoids, all pigments are reported as nmol g−1 organic matter.
Total microcystins were measured using the total microcystin/nodularin ADDA enzyme-linked immunosorbent assay (ELISA) kit commercially available from Eurofins (https://abraxis.eurofins-technologies.com). Total microcystins were extracted from dried sediment samples by combining the methods provided by Eurofins for soils and from a previous study (Waters et al. 2021). A mixture of 75% methanol and 25% water acidified with trifluoracetic acid, was used as the extraction solution. Samples were twice extracted with the acidified methanol solution, sonicated in a water bath for 20 min, and centrifuged. The supernatants were collected and combined. Combined supernatants were concentrated to ~ 1.5 mL by evaporation under controlled heat and nitrogen flow on an Organomation N-EVAP 111 Nitrogen Evaporator. Extracts were diluted up to 6 mL with water, sonicated, and vortexed to dissolve all contents. Extractants were then passed through Strata-X 33 µm polymeric reversed-phase columns to remove interferents. Strata-X columns were conditioned with 6 mL of methanol followed by 6 mL of water. The sample was passed through the column, and then the column was rinsed with 2 mL of 5% methanol. All volumes of the rinse, sample solution, and methanol rinse were discarded. The sample was eluted through the column with 9 mL of 90% acetonitrile. Eluted sample solutions were again evaporated under heat and nitrogen flow and reconstituted with 1.5 mL of water to a consistent volume for analysis with the ELISA kits. Microcystins were reported as ng microcystin g−1 organic matter.
Samples for radiometric dating were packed in plastic test tubes, sealed with epoxy, and stored for > 21 days for samples to reach 226Ra/210Pb equilibrium. An ORTEC Intrinsic Germanium Detector was used to measure 210Pb, 214Bi (i.e. 226Ra), and 137Cs activity by direct gamma counting (Appleby et al. 1986). 226Ra activity is used as a proxy for “supported” 210Pb activity—the activity generated by in situ radium in the sediments. “Unsupported” 210Pb activity is calculated as the difference between total 210Pb activity and supported 210Pb activity. Atmospheric deposition of 210Pb occurs at a constant rate and, assuming that the lake morphology remains constant over time, can be used to assign dates to sediment samples through the Constant Rate of Supply Model (CRS) (Appleby and Oldfield 1983).
Results
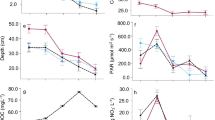
The 165-cm sediment core consisted of dark organic sediments throughout the core with highly flocculent sediments in the top 16 cm. Excess 210Pb steadily decreased down core and the CRS provided dates for the top 64-cm of the core spanning the last ~ 150 years and showed that the entire Lake Carlton sediment record spans > 150 yrs (Fig. 2). This dating model was further validated by similar stratigraphic changes in non-volatile solids between the Lake Carlton and Lake Beauclair (Schelske et al. 2005) cores (Fig. 3).
Comparison of sedimentary records from Lake Carlton and Lake Beauclair (LB2-99) for TC:TN ratios, TP, and LOI from Lake Carlton and TC:TN ratios, TP, and LOI data from Lake Beauclair from Schelske et al. (2005). The dotted lines on each figure represent 1947, which is believed to be the date when Lake Apopka turned into an algal-dominated system
In Lake Carlton, a sharp increase in P was observed ~ 1900 CE (61 cm) followed by a steady increase to the top of the core. Maximum P concentration was 2.4 mg g−1 in 2015 CE. N had a maximum of 3.66% around 2015 CE, with a potential outlier of 5.21% predating the 210Pb record at 76 cm (Fig. 4). Maximum C concentrations of 36.25% also predate the 210Pb record at a depth of 84 cm (Fig. 4). Molar nutrient ratios exhibited shifts with N:P values before anthropogenic nutrient loading ranging from 27.6 to 9.7 before 1900 and then from 8.3 to 3.7 after excess P is introduced (Fig. 4). C:N experienced a steady decline throughout the core with historic values decreasing from 17 to 12.6 before ~ 1900 CE and decreasing from 12.7 to 10.5 after anthropogenic impacts to the top of the core (Fig. 4).
Sedimentary records for organic content (as loss on ignition, %), non-volatile solids (%), phosphorus (mg g−1), carbon (%), nitrogen (%), carbon to nitrogen ratio, and nitrogen to phosphorus ratio versus depth for Lake Carlton. Ratios are reported as molar ratios. Dates are determined for excess 210Pb measurements and the CRS model
All photosynthetic pigment records indicate phytoplankton abundance increased throughout the record with a sharp spike in productivity that corresponds with increases in P at 61 cm or ~ 1900 CE (Fig. 4). Following this increase, all photosynthetic pigments and total fluorescence spiked at 61 cm. Chl-a showed peaks at 61 cm (~ 1900 CE) and 44 cm (~ 1960 CE) with the highest values in the top of the core. Diatoxanthin, specific for diatoms, reached its maximum concentration at 40 cm (~ 1970 CE) and decreased to the present day. Alloxanthin, specific for cryptophytes, had several peaks (61 cm, 44 cm) including a maximum at 24 cm in ~ 1992 CE. Cyanobacteria pigments (aphanizophyll and echinenone) exhibited similar peaks and troughs throughout the core, and both reached maximums at 20 cm in ~ 1997 CE. Canthaxanthin, an additional cyanobacteria pigment, followed similar trends to diatoxanthin as it reached its maximum concentration at 40 cm (~ 1970 CE) and decreased to the present day. Phytoplankton-community structure shifted towards the top of the core, moving from diatom dominance to cyanobacterial dominance after ~ 1980 CE at a depth of 36 cm (Fig. 5) as indicated by stratigraphic change from diatoxanthin (diatoms) and aphanizophyll (N-fixing cyanobacteria). Low amounts of microcystin were detected through the core with a large increase in recent sediments corresponding to ~ 2005 CE (Fig. 5). Detectable amounts of microcystin were present before large-scale human disturbance at 85 cm.
Photosynthetic pigment records versus depth from Lake Carlton: total florescence, diatoxanthin (diatoms), alloxanthin (cryptophytes), canthaxanthin (cyanobacteria), chlorophyll a (total primary producers), echinenone (cyanobacteria), aphanizophyll (N2-fixing cyanobacteria), and microcystin concentration (Leavitt and Hodgson 2001). Pigments are reported in nmol pigment g−1 organic matter and microcystins are reported in µg g−1 organic matter. Dates are determined for excess 210Pb measurements and the CRS model
Discussion
The sediment record from Lake Carlton demonstrates the increased P loading and subsequent increase in algal production coincided with a period of canal construction beginning in 1893 CE. As a result, the P inputs impacting Beauclair from Apopka (Schelske et al. 2005) further impacted Carlton on a very similar timescale. Schelske et al. (2005) used LOI data from Lake Apopka and Lake Beauclair to demonstrate the hydrologic connectivity between these two lakes to construct an age model for Lake Apopka. The sediment record from Lake Carlton exhibits the same trends in LOI, validating P source assumptions that nutrient inputs entering Lake Carlton originated from Lake Apopka suggesting that hydrologic connectivity can have a cascading impact to multiple downstream lakes. These hydrologic connections between Lake Carlton and Lake Beauclair have been documented by the Florida DEP and the Lake County Water Authority (Amec 2018) through their water quality monitoring program, but our data show the long-term temporal connection following canal construction. Total primary producer abundance and diagnostic photosynthetic pigment concentrations suggest several periods of increased primary producer abundance throughout the core in response to both P originating from Lake Apopka and more recent change in phytoplankton-community structure. The first of these peaks coincides with the period of canal construction ~ 1900 CE (Fig. 5) and corresponded to an increase in all pigments demonstrating a dominance by diatoms (diatoxanthin) and some cyanobacteria (canthaxanthin). After ~ 1930 CE, pigment concentrations briefly decreased then increased to form another peak around ~ 1970 CE. The 1970 CE pigment peak indicated changes in phytoplankton-community structure from diatom dominance to cyanobacterial dominance. These variations in pigment concentrations correspond to variations in P concentrations throughout the core. While the original increase in P loading from the Apopka-Beauclair Canal construction rapidly increased primary production in Lake Carlton, there is a significant lag time between increased nutrient loading and the change from diatom to cyanobacterial dominance (Fig. 5). This lag between increased nutrient loading and cyanobacterial dominance is consistent with other work investigating cyanobacterial dominance and alternative equilibria in shallow lakes (Scheffer et al. 1993; Kenny et al. 2002; Waters et al. 2015, 2021).
Nutrient inputs from lakes Apopka and Beauclair into Lake Carlton
According to the Mount Dora Historical Society (https://ci.mount-dora.fl.us/317/History-of-Mount-Dora), a railroad line was extended to Mount Dora in the mid-1890s CE. This rail-line extension spurred the construction of a network of canals—including the Apopka-Beauclair Canal and the Carlton Cut—throughout the Harris Chain of Lakes, which allowed the transportation of goods, tourists, and subsequently P. The increased agricultural activities spurred a dramatic increase in nutrient loading and stimulated primary production in Lake Apopka ~ 1940 and downstream. Increased wastewater drainage from muck farming practices on the Northern end of Lake Apopka led to increased P loading into the lake and lakes downstream (Schelske et al. 2005).
Previous work from Schelske et al. (2005) was used to corroborate our dating model and stratigraphic changes within the Lake Carlton core. Schelske et al. (2005) used sediment-core analysis to connect nutrient loading in Lake Beauclair to the excess nutrients in Lake Apopka. Stratigraphic changes in LOI, P, and C:N ratios showed Lake Apopka has a direct influence over the nutrient dynamics within Lake Beauclair. Data from Schelske et al. (2005) were compared to the core recovered from Lake Carlton (Fig. 3). Both Lake Carlton and Lake Beauclair exhibit similar peaks in LOI ~ 1950 CE, as well as slight decreases in P and increases in C:N ratios. Through these similarities in stratigraphic changes in LOI, P, and C:N ratios, we can demonstrate direct influences from Lake Beauclair and Lake Apopka on Lake Carlton.
Other nutrient data support these anthropogenic alterations as well. C:N ratios display a significant difference (p = 2.97e-8) between historic sediments prior to 1893 CE and sediments deposited after connection to Lake Apopka in 1893 CE. Trends in C:N sediment records from Lake Carlton match records presented in previous work (Schelske et al. 2005; Waters et al. 2015). These decreasing C:N ratios suggest a possible change from a macrophyte-dominated system to increased phytoplankton production (Wetzel 2001; Meyers and Terranes 2001). Kenney et al. (2010) showed that many lakes within the Harris Chain of Lakes experienced similar changes to C:N following the construction of the canal network. While Carlton was not in the direct chain of lakes, it was connected to Beauclair after 1890 CE, and similar ecological changes occurred within other members of the Harris Chain.
These changes are also made evident by the decrease in N:P ratios. Historic N:P values decrease from 25 to 10 before leveling off around ~ 3 after 1950 CE. Alterations to nutrient ratios suggest changes in the source and volume of organic matter within Lake Carlton. With large increases in P loading, N:P ratios would decrease. Also, due to its gaseous component, N burial in the sediment does not typically result in long-term storage. N deposition in sediments can be skewed by other external factors, including rates of microbial processing that are not typically measured in paleolimnological studies (Finlay et al. 2013). Low N:P ratios could also favor cyanobacteria production in the water column as there are many N-fixing genera of cyanobacteria (Wetzel 2001).
Primary producer alterations from nutrient inputs
While P inputs and the initial primary producer increases appear to be linked to the Apopka-Beauclair Canal, Lake Carlton experienced a significant alteration from diatom dominance to cyanobacteria dominance around ~ 1970 CE. The change from diatom to cyanobacteria dominance has been shown in other pigment studies on tropical and subtropical lakes (Waters et al. 2015, 2021). Cyanobacteria respond to disturbances better than diatoms and usually develop dominance in highly disturbed systems (Paerl and Valerie 2012). As P loading increased in the system, many lakes within the Harris Chain reached tipping points causing regime shifts to cyanobacteria dominance (Kenney et al. 2002, 2010). As higher trophic status is achieved through increased P loading and algal production, inorganic N uptake is stimulated (Schindler 2012). As phytoplankton die, they settle to the bottom and act as a labile source of energy for heterotrophic metabolism (Tartari and Biasci 1997). This increased microbial activity decreases dissolved oxygen concentrations, which increases denitrification (Seitzinger et al. 2006). This continual P loading (Fig. 4), coupled with increased denitrification, gives nitrogen-fixing cyanobacteria a competitive advantage over other primary producers (Paerl and Huisman 2008). Low N:P ratios (Fig. 4) and increases in aphanizophyll concentrations (nitrogen-fixing cyanobacteria; Leavitt and Hodgson 2001) (Fig. 5) support the inference that nitrogen limitation and cyanobacteria advantages could have led to the primary producer alterations exhibited in Lake Carlton. This positive relationship between N-fixing cyanobacteria (aphanizophyll concentrations) and P is strong (r2 = 0.914, n = 42; Table S-1). The significant lag between initial nutrient loading and cyanobacterial dominance is consistent with other work investigating cyanobacterial dominance and alternative equilibria in shallow lakes (Scheffer et al. 1993; Kenny et al. 2002; Waters et al. 2015, 2021).
Cyanotoxin production in Lake Carlton
Cyanotoxins show a drastic increase in recent sediments of Lake Carlton from ~ 2005 to ~ 2015 CE. Though there are smaller increases in cyanotoxin concentration throughout the core beginning around the time of canal construction, this large increase in the upper sections of the core is an order of magnitude higher than other observed concentrations. There is a significant lag between peak-cyanobacteria abundance and cyanotoxin concentration, which has been shown in other lake systems (Waters 2016; Waters et al. 2021). Echinenone is a photosynthetic pigment thought to be associated with cyanobacteria capable of producing microcystin (Leavitt and Hodgson 2001; Waters et al. 2021), and our results show a distinct relationship between microcystin and echinenone in Lake Carlton (r2 = 0.47, p = 0.0096: Table S-1). Though the sharp drop-off in toxin concentration resembles a degradation curve, microcystins are chemically stable and deposition has been shown to occur over 1000 years in other studies (Waters et al. 2021). Their toxicity remains after boiling (Harada 1996) and can persist for years when stored dry and at room temperature (Metcalf et al. 2012). Rapid chemical hydrolysis is also unlikely outside of laboratory conditions (Harada 1996). Likewise, other records covering similar time periods (~ 150 years) that measure microcystins have demonstrated similar trends with dramatic increases in the last few decades (Efting et al. 2011; Zastepa et al. 2017). When considering longer timescales, previous studies have also produced cyanotoxin records spanning thousands of years (Waters 2016; Waters et al. 2021). While sedimentary cyanotoxin interpretations are still being developed, these measurements of total microcystins suggest that cyanotoxin production in Lake Carlton began with the onset of P inputs from the canal but reached higher levels in only the last few decades.
Implications for hydrologically connected lake systems
This work shows the importance of hydrologic connectivity and how it can impact nutrient dynamics and primary productivity in connected lake systems. With the construction of the Apopka-Beauclair Canal, excess nutrient pollution was able to move downstream from Lake Apopka and into Lake Beauclair and finally to Lake Carlton. These nutrient inputs immediately stimulated primary production, and coupled with local sources, caused hypereutrophic conditions in Lake Carlton resulting in a primary producer-community shift. As nutrient dynamics changed over time, low N:P ratios favored cyanobacteria and triggered a replacement from diatoms to cyanobacteria as the dominant primary producers in the system. This cyanobacteria dominance gave rise to increased concentrations of cyanotoxins, which can be hazardous to lake users and lake biota. However, decreases in photosynthetic pigments and nutrient concentrations at the top of the core indicate that local nutrient-pollution-mitigation programs may be working. Ongoing restoration efforts in Lake Apopka and the Lake County Nutrient Reduction Facility located on the Apopka-Beauclair Canal could help mitigate the excess nutrient pollution that has negatively impacted water quality in the Harris Chain of Lakes for more than five decades. Florida DEP has already documented noticeable water-quality improvement in lakes Beauclair and Carlton since the installation of the water-treatment facility. TP concentration has decreased significantly in Lake Carlton over the past 19 years (R2 = 0.29, p < 0.01) (Amec 2018). The annual TP geometric mean was highest in 1999 at 87 μg/L and lowest in 2010 at 35 μg/L (Amec 2018). By treating water from the Apopka-Beauclair Canal and removing sedimentary P Lake Beauclair, water quality within Lake Carlton is improving.
Phosphorus mobility within sediment
P is known to be mobile in sediments (Ginn et al. 2012; Torres et al. 2014), however complex stratigraphic records can demonstrate a lack of post-depositional mobility. If P mobility was significant, P concentrations should equilibrate and minimize any large, stratigraphic changes down the core (Kenny et al. 2016). P records have been used extensively to investigate eutrophication in subtropical lakes (Kenny et al. 2002; Schelske et al. 2005).
Conclusions
Rapid influx of non-volatile solids and nutrients correspond with the onset of enhanced hydrologic connectivity between lakes Carlton, Beauclair, and Apopka. Hypereutrophic conditions in Lake Apopka had downstream effects on lakes Beauclair and Carlton. The increased availability of nutrients stimulated primary production in Lake Carlton and ultimately led to a cyanobacteria-dominated primary producer community. This shift is evident in changes in nutrient ratios and fossil pigment records.
Increased P loading and increased denitrification strengthened the competitive advantages of nitrogen-fixing cyanobacteria. This positive feedback loop strengthens cyanobacterial dominance of the phytoplankton community, which leads to a greater prevalence of HABs and the possibility for cyanobacteria-toxin production, as many N-fixing cyanobacteria are known to produce them. This selection for cyanobacteria is problematic for lake managers as cyanobacteria can outcompete other phytoplankters and increase turbidity, which shades aquatic plants and macrophytes. Cyanobacteria dominance can also be concerning to lake users, as some genera of cyanobacteria are known to produce toxins hazardous to human health (Carmichael 1992). The resilience of cyanobacteria communities in the Harris Chain of Lakes has been shown through intense management efforts focused on Lake Apopka where cyanobacteria are still the dominant primary producer and macrophytes have proven to be difficult to reestablish despite this concentrated effort (Schelske et al. 2005; Waters et al. 2015). This increased potential for cyanotoxin production is a concern for lake users and managers, which has led to widespread restoration efforts, which may be effective at mitigating the downstream impacts from Lake Apopka.
Data availability
The data collected during this project are available upon request.
References
Amec Foster Wheeler Environment & Infrastructure, Inc. (2018) Lake Carlton Hydrologic/Nutrient Loading Study
Appleby PG, Oldfield F (1983) The assessment of 210Pb data from sites with varying sediment accumulation rates. Hydrobiologia 103:29–35
Appleby PG, Nolan PJ, Gifford DW, Godfrey MJ, Oldfield F, Anderson NJ, Battarbee RW (1986) 210Pb dating by low background gamma counting. Hydrobiologia 143:21–27
Bachmann RW, Hoyer MV, Canfield DE (2000) The potential for wave disturbance in shallow Florida lakes. Lake Res Manag 16(4):281–291
Brenner M, Keenan LW, Miller SJ, Schelske CL (1999) Spatial and temporal patterns of sediment and nutrient accumulation in shallow lakes of the upper St. Johns river basin. Florida Wetl Ecol Manag 6:221–240
Carmichael WW (1992) Cyanobacterial secondary metabolites – the cyanotoxins. J Appl Bacteriol 72:445–459
Efting AA, Snow DD, Fritz SC (2011) Cyanobacteria and microcystin in Nebraska (USA) Sane Hills Lakes before and after modern agriculture. J Paleolimnol 46:17–27
Finlay JC, Small GE, Sterner RW (2013) Human influences on nitrogen removal in lakes. Science 342:247–250
Fisher MM, Brenner M, Reddy KR (1992) A simple, inexpensive piston corer for collecting undisturbed sediment water interface profiles. J Paleolimnol 7:157–161
Fulton RS, Paerl HW (1987) Toxic and inhibitory effects of the blue-green alga microcystis aeruginosa on herbivorous zooplankton. J Plankton Res 9:837–855
Ginn BK, Ruhland KM, Young JD, Hawryshyn J, Quinlan R, Dillon PJ, Smol JP (2012) The perils of using sedimentary phosphorus concentrations for inferring long-term changes in lake nutrient levels: comments on Hiriart-Bear et al., 2011. J Great Lakes Res 38:825–829
Hakanson L, Jansson M (1983) Principals of lake sedimentology. Springer, New York. 316
Harada KI (1996) Chemistry and detection of microcystins. In: Watanabe MF, Harada KI Carmicheal WW, Fujiki H (eds) Toxic Microcystis, 1st edn. CRC Press, Boca Raton (FL), pp 103–104
Heisler J, Gilbert PM, Burkholder J, Anderson D, Cochlan W, Dennison W, Gobler C, Dortch Q, Heil C, Humphries E, Lewitus A, Magnien R, Marshall H, Sellner K, Stockwell D, Stoecker D, Suddleson M (2008) Eutrophication and harmful algal blooms: a scientific consensus. Harmful Algae 8(1):3–13
Ho JC, Michalak AM, Pahlevan N (2019) Widespread global increase in intense lake phytoplankton blooms since the 1980s. Nature 574:667–670
Kenney WF, Waters MN, Schelske CL, Brenner M (2002) Sediment records of phosphorus-driven shifts to phytoplankton dominance in shallow Florida lakes. J Paleolimnol 27:367–377
Kenney WF, Brenner M, Curtis JH, Schelske CL (2010) Identifying sources of organic matter in sediments of shallow lakes using multiple geochemical variables. J Paleolimnol 44:1039–1052
Kenny WF, Brenner M, Curtis JH, Arnold TE, Schelske CL (2016) A holocene sediment record of phosphorus accumulation in shallow Lake Harris, Florida (USA) offers new perspectives on recent cultural eutrophication. PLOS ONE 11(1):e0147331
Leavitt PR, Hodgson DA (2001) Sedimentary pigments. In: Tracking environmental change using lake sediments, terrestrial, algal, and siliceous indicators, vol. 3. In: Smol JP, Birks HJP, Last WM (eds). Kluwer, Dordrecht, pp. 295–325.
Metcalf JS, Richer R, Cox PA, Codd GA (2012) Cyanotoxins in desert environments may pose risk to human health. Sci Tot Env 421:118–123
Paerl HW, Huisman J (2008) Blooms like it hot. Science 320(5872):57–58
Paerl HW, Huisman J (2009) Climate change: a catalyst for global expansion of harmful cyanobacterial blooms. Env Microbiol Rep 1(1):27–37
Paerl HW, Valerie JP (2012) Climate change: links to global expansion of harmful cyanobacteria. Water Res 46(5):1349–1363
Scheffer M, Hosper SH, Meijer M-L, Moss B, Jeppesen E (1993) Alternative equilibria in shallow lakes. Trend Ecol Evol 8(8):275–279
Scheffer M, van Nes EH (2007) Shallow lakes theory revisited: various alternative regimes droven by climate, nutrients, depth and lake size. Hydrobiologia 584:455–466
Schelske CL, Lowe EF, Battoe LE, Brenner M, Coveney MF, Kenney WF (2005) Abrupt biological response to hydrologic and land-use changes in Lake Apopka, Florida, USA. Ambio 34(3):192–198
Schindler DW (1977) Evolution of phosphorus limitation in lakes. Science 196:260–262
Schindler DW (2012) The dilemma of controlling cultural eutrophication of lakes. Proc R Soc 279:4322–4333
Seitzinger S, Harrison JA, Böhlke JK, Bouwman AF, Lowrance R, Peterson B, Tobias C, Van Drecht V (2006) Denitrification across landscapes and waterscapes. Ecol Appl 16(6):2064–2090
Steffen W, Crutzen PJ, McNeil JR (2007) The Anthropocene: are humans now overwhelming the great forces of nature? Ambio 36:614–621
Tartari G, Biasci G (1997) Trophic status and lake sedimentation fluxes. Air Water Soil Pollut 99:523–531
Torres IC, Turner BL, Reddy KR (2014) The chemical nature of phosphorus in subtropical lake sediments. Aquat Geochem 20:437–457
Waters MN (2016) A 4700-year history of cyanobacteria toxin production in a shallow subtropical lake. Ecosystems 19:426–436
Waters MN, Schelske CL, Kenney WF, Chapman (2005) A.D. The use of sedimentary algal pigments to infer historic algal communities in Lake Apopka. Florida. J Paleolimnol. 33(1):53–71
Waters MN, Piehler MF, Rodriguez AB, Smoak JM, Bianchi TS (2008) Shallow lake trophic status linked to late Holocene climate and human impacts. J Paleolimnol 42:51–64
Waters MN, Schelske CL, Brenner M (2015) Cyanobacteria dynamics in shallow Lake Apopka (Florida, USA) before and after the shift from a macryophyte to a phytoplankton-domianted state. Freshw Biol 60(8):1571–1580
Waters MN, Brenner M, Curtis JH, Romero-Oliva CS, Dix M, Cano M (2021) Harmful algal blooms and cyanotoxins in Lake Amatitlan, Guatemala, coincided with ancient Maya occupation in the watershed. PNAS 118(48):1–8
Webster BC, Waters MN, Golladay S (2021) Alterations to sediment nutrient deposition and transport along a six reservoir sequence. Sci Tot Env 785:147246
Wetzel RG (2001) Limnology: lake and river ecosystems, 3rd edn. Elsevier, San Diego
Whitmore TJ, Brenner M, Schelske CL (1996) Highly variable sediment distribution in shallow, wind-stressed lakes: a case for sediment-mapping surveys in paleolimnological studies. J Paleolimnol 15:207–221
Zastepa A, Teranu ZE, Kimpe LE, Blais JM, Gregory-Eaves I, Zurawell RW, Pick FR (2017) Reconstructing a long-term record of microcystins from the analysis of lake sediments. Sci Total Env 579:893–901
Acknowledgements
Student workers, research assistants, and lab technicians made this project possible. Thank you for your help, both in the field and in the lab.
Funding
This project was supported through funding provided by the National Science Foundation (Grant: 1941568).
Author information
Authors and Affiliations
Contributions
Clift and Waters wrote the main manuscript text and Clift prepared the figures. Both authors reviewed the manuscript before submittal.
Corresponding author
Ethics declarations
Conflict of interest
The Authors have no competing interests as defined by Springer, or other interests that might be perceived to influence the results and/or discussion reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Clift, T.L., Waters, M.N. Paleolimnological evidence for primary producer change linked to hydrologic connectivity and human impacts in Lake Carlton, Florida, USA. J Paleolimnol 72, 35–48 (2024). https://doi.org/10.1007/s10933-024-00318-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10933-024-00318-y