Abstract
This study provides a new record of nitrogen (N) deposition and ecological response at a remote, high-elevation lake within the ecologically fragile Three River Headwaters Region, northwest China, from the early 20thCentury to the past decade. A multi-proxy investigation of a lake sediment core including analyses of chironomids (non-biting midge), lake sediment geochemistry (δ15N, δ13C, TN, TOC, OC:TN), pollen, non-pollen palynomorphs and 210Pb-dating. Results were compared with regional pollution forcings, including fertiliser application, atmospheric nitrate and dust deposition, and a localised multiscale air quality modelling system for N-deposition. Our data reflect a mesotrophic lake for the majority of the 20th Century with minimal anthropogenic impact. A shift in the lake conditions is evident after 1985 with increased nutrient enrichment. Low δ15N, OC:TN and increasing TN values, together with an increase in chironomid taxa associated with eutrophic conditions and macrophytes are exhibited in the record, particularly since 2002. These changes are consistent with nutrient loading causing a change in trophic status. Redundancy analysis (RDA) and partial RDAs verified TN as an important driver behind the shift in chironomid community composition in recent decades, explaining 22.2% of the variance on its own and 16.9% with other environmental variables partialled out. While temperature change since 1960 was not a primary control on the chironomid community of Lake Bander, a step-change increase in summer temperatures since the 1990s coincided with the disappearance of cold stenotherms from the record and seems to have exacerbated the shift to more productive conditions. This study highlights the reach of airborne N from modern agricultural and industrial activity to remote locations, and demonstrates the utility of palaeolimnological techniques as part of modern ecosystem assessment for conservation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Qinghai-Tibetan Plateau, with an average elevation of over 4000 m a.s.l., is the largest high-altitude region on Earth. Due to its geographic location and cold climatic conditions, the plateau is classified as one of the most sensitive, remote and fragile ecosystems in the world, often referred to as ‘the roof of the world’ or the ‘third pole’ (Li et al. 2012). The Three River Headwaters Region (TRHR), also known as the Sanjiangyuan region, comprises the hinterland and main body of the Qinghai-Tibetan Plateau, covering a total area of 363,000 km2 (Zhang et al. 2014). The TRHR is the headwater of Asia’s largest rivers, with the Yangtze, Yellow and Lancang-Mekong Rivers providing freshwater to over a billion people (Qu et al. 2019).
Despite its remote location, the fragility of the TRHR ecosystems continues to be threatened by global warming, local population pressures and local and regional agricultural and industrial activities (Zhou et al. 2021). Since the 1980s, pastoral farming (grazing) in the TRHR has developed rapidly, with animal husbandry utilising approximately 60% of the land cover (Luo et al. 2017). Livestock numbers have tripled since 1978 (Du et al. 2004). Increased synthetic fertiliser application has been recorded in the Qinghai region from the late 1980s (National Bureau of Statistics of China), with the majority of applications from April to June (Zhang et al. 2011). Though yet to be recorded in the TRHR region specifically, increased fertiliser application has been shown to result in airborne transport of gaseous and fine particulate nitrogen to remote areas of the Tibetan Plateau, resulting in increased N-deposition. Such evidence of increased agriculturally-derived N-deposition has been recorded in an ice core record from 6000 m elevation in the middle of the Tibetan Plateau (Li et al. 2020b). The region is also impacted by extra-regional pollution brought by the Southwest and East Asian monsoons in summer and westerly airflow, which can carry pollutants from agricultural, industrial and vehicle emissions atmospherically over long distances (Hu et al. 2014; Liu et al. 2015). Increased atmospheric dust deposition, linked to changes in climate and anthropogenic activity, has been recorded in Tibetan ice cores (Li et al. 2020b; Thompson et al. 2000; Wu et al. 2013) and lake sediment records (Wan et al. 2016) in the region. Lastly, the Qinghai-Tibetan Plateau is particularly sensitive to global warming (Liu and Chen 2000), with warmer temperatures leading to grassland degradation (Fayiah et al. 2020), fluctuations in lake levels (Liao et al. 2013) and enhanced algal production in lake ecosystems (Kang et al. 2019).
Lakes can act as excellent records of nutrient deposition as they are typically nutrient-limited systems that respond readily to increased nutrient influx (Carpenter 2005). Synthetic fertilisers can stimulate increased within-lake productivity (Carpenter et al. 1998), while atmospheric nitrogen (NO3) and dust deposition can further contribute as a source of nitrogen (N), often resulting in lake eutrophication even in remote, high-elevation lake systems (Chen et al. 2018; Wu et al. 2021). Global warming can also exacerbate lake eutrophication through increased stratification, reduced water clarity and increased risk of cyanobacterial blooms (Jeppesen et al. 2010), and its effects on changing lake productivity must also be assessed in regions impacted by rapid warming (Guo et al. 2013).
This study aims to assess the potential ecological impact of N-pollution in the fragile TRHR, using a lake sediment core from a remote, high-elevation catchment within the region. A multi-proxy approach of chironomid subfossils, lake sediment geochemistry (δ15N, δ13C, TN, TOC, OC:TN) and fossil pollen analyses were used to determine nutrient influx from N-deposition, landscape change and the associated ecological lake response from the early 1900s to the past decade. Chironomid (Diptera: Chironomidae) subfossils are a versatile biological indicators in palaeoenvironmental reconstruction, due to their sensitivity to changing limnological conditions, worldwide distribution, ubiquitous abundance and low mobility which generates a local signal (Brooks et al. 2007; Walker 2001). Chironomid communities are affected both directly and indirectly by a variety of environmental variables including lake water pH, depth, temperature and nutrient enrichment (Eggermont and Heiri 2012). Stable isotopic analysis of carbon and nitrogen provides information on nutrient influx, potential nutrient sources and lake response (Cohen 2003; Taylor et al. 2018), while pollen analysis infers changing landscape cover (vegetation) and within-lake conditions. The results of the palaeolimnological reconstruction were compared with records of regional environmental pollution and a localised multiscale air quality modelling system for N-deposition to determine dominant pollution sources through time.
Study site
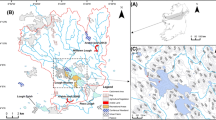
Lake Bander (34° 14′ 24.60″ N, 92° 06′ 13.35″ E) is a large (4.4 km2), high-elevation lake (4583 m a.s.l) located within an experimental zone (defined as a normal protected area, consideration is given to both protection and utilization) of Sanjiangyuan National Park (SNP) in the TRHR, western Qinghai Province, China (Fig. 1). The lake lies 30 km west of the town Tanggulashan (‘Dangla Town’) of Golmud Municipality, the Qinghai-Tibet National Highway (G109) and the Qinghai-Tibet Railway. The shallow, polymictic (well-mixed) freshwater lake has a maximum water depth of 4 m and is fed mainly by precipitation and glacial runoff. Two small streams enter the lake on the western and southwestern side, with two minor outflows to the northeast and southeast, the latter of which flows into the Tuotuo River, the upper reach of the Yangtze River.
Map A showing the location of the Three Rivers Headwaters Region (TRHR), in Qinghai Province, China and the location of map B (red square). 1 = Yellow River, 2 = Yangtze River, 3 = Lancang-Mekong River; elevation shades show 0–1000 m (dark gray), 1000–4500 m (light gray), > 4500 m (white). Map B illustrates the study site location of Lake Bander within the TRHR including, rivers, lakes, glaciers, the main road (G109), nearby town (Tanggulashan) and the Tuotuohe River Meteorological Monitoring Station. The black rectangle indicates the location of map C as follows. Map C) shows Copernicus Sentinel-2 imagery (collected 19 Aug 2020) of the Lake Bander catchment with a yellow star marking the coring location of BL-B
The main lithology for the study area includes a Triassic group surrounded by Carboniferous-Permian and Eocene-Miocene strata (Qian et al. 2014). Lake Bander itself lies on a clastic sedimentary rock formation-complex which includes Triassic sandstone, shale and limestone (Jiang et al. 2015; Qian et al. 2014). The surrounding catchment vegetation is dominated by alpine steppe, a natural alpine grassland which occupies a large portion of the interior region of the Qinghai-Tibetan Plateau at elevations of 4400–5400 m a.s.l. (Lu et al. 2011). The vegetation distribution in the Qinghai-Tibetan Plateau is largely controlled by summer monsoon rainfall coming from the southeast (Lu et al. 2011). The area is characterised by a cold, dry climate with a mean annual temperature of − 4.9 °C (max. 6.6 °C; min.–16.7 °C) and a mean July temperature of 6.6 °C (Harris et al. 2020). Average annual precipitation is 214 mm and is predominantly recorded between the months of June and September (Harris et al. 2020). In the past decade, Lake Bander has become an important breeding site for bar-headed geese (Anser indicus), a migratory bird listed as ‘vulnerable’ due to wetland degradation (BirdLifeInternational.org; EBird.org). Green River’s monitoring programme recorded up to 2800 bar-headed geese at Lake Bander in 2015 (Green-River.org).
Materials and methods
Sediment core
In July 2016, four parallel sediment cores (BL-A, BL-B, BL-C, BL-D) were extracted from Lake Bander using a 5-cm-diameter gravity corer. All analyses were conducted on the BL-B core which measured 34 cm in length, as it displayed the most stable sedimentation profile (i.e. based on loss-on-ignition). Although all cores were of similar length, cores BL-A, BL-C and BL-D (with less stable loss-on-ignition results) were excluded from further analysis due to potential sediment mixing in this large, relatively shallow lake. The core was sectioned in 1-cm intervals in the field and later transported to Sichuan University, then to the Palaeoenvironmental Research Unit, National University of Ireland Galway (NUIG) for storage and analysis. Samples are reported as top depths moving forward.
Dating model
Twelve samples (BL-0, 1, 2, 4, 6, 8, 11, 14, 17, 21, 25 and 31 cm) were measured by alpha-spectroscopy 210Pb analysis by Chronos Scientific (chronosscientificinc.ca). In preparation for 210Pb dating, samples were air-dried, weighed and sieved through a 100-µm mesh. The age-depth model was created in Bacon 2.3.4 (Blaauw and Christen 2011) based on constant rate of supply (CRS)-derived 210Pb dates. Markov Chain Monte Carlo (MCMC) iterations for the model are as follows: accumulation shape = 0.5, accumulation mean = 5 yr cm−1, memory strength = 4, memory mean = 0.7.
Chironomid analysis
Chironomid analysis was completed for all thirty-four contiguous samples of core BL-B, at 1-cm resolution (0–33 cm). Between 1 and 5 cm3 of wet sediment was warmed to 30 °C in a 10% KOH solution and deflocculated for 30 min. The sediment was then passed through a 90-µm mesh following standard procedures outlined in Walker (2001). The remaining solution was poured into a Bogorov plankton counting tray and sorted under a Motic® SMZ Series dissection microscope (10–40 × magnification). An average of 54 chironomid head capsules per sample (Quinlan and Smol 2001), with the exception of 16 cm (38 head capsules), were removed with fine forceps and permanently mounted on slides with Entellan®. A Motic® B3 Professional Series compound microscope at 100–400 × magnification was used for identification of chironomid subfossils. Taxa were identified following Zelentsov (1989), Rieradevall and Brooks (2001), Brooks et al. (2007) and Andersen et al. (2013). A chironomid percentage diagram was created using the program C2 version 1.7.7 (Juggins 2016). Zonation was performed with sum-of-squares partitioning on percentage chironomid data for all taxa using psimpoll version 4.27 (Bennett 2009–1993).
Geochemical analyses
Twenty-six contiguous samples (0–25 cm) were analysed on the datable portion of the core for stable isotope ratios of nitrogen (δ15N) and carbon (δ13C), and their elemental content (%N, %C) by EA-IRMS using a ThermoFinnigan Delta XP interfaced to a Costech ECS 4010 via a ThermoFinnigan MAT Conflo IV at the Isotope Biogeochemistry Laboratory, University of Hawaiʻi at Mānoa. Sediment samples of 1 cm3 were dried for 24 h at 100 ˚C, homogenized using a Retsch MM200 Mixer Mill with stainless jars and balls, and sieved through a 250-μm mesh. Lake Bander is situated within a drainage basin that is rich in carbonate rock (Jiang et al. 2015; Qian et al. 2014); therefore, removal of inorganic carbon was important to see changes in organic carbon cycling. Carbonates were removed following a modified version of Harris et al. (2001) in which 2–5 mg of homogenized lake sediment was HCl-fumigated in silver capsules. OC:TN ratios were calculated as the mass ratio of organic carbon (OC) to total nitrogen (TN). Stable isotope ratios of nitrogen and carbon are reported as conventional delta values relative to atmospheric N2 and V-PDB, respectively. Accuracy and precision were 0.2 ‰ or better relative to reference materials analyzed every 10 samples. Quantification and correction of isotope values was achieved using internal lab reference materials (glycine and acetanilide) that are characterised against international certified reference materials (NBS18, NBS19, NIST 1547 NIST3, USGS32, USGS34, USGS35).
Loss-on-ignition
Loss-on-ignition (LOI) analysis was completed on all 34 samples at 1-cm intervals. Analysis followed standard procedures from Heiri et al. (2001). Sediment samples of 2 cm3 were sequentially ashed at 550 °C and 950 °C to determine organic (LOI550) and carbonate (LOI950) content of samples.
Pollen analysis
Pollen analysis was carried out on a total of 15 samples at varying intervals (0–33 cm). Individual pollen samples of 2 cm3 were taken at 1-cm intervals in the upper 5 cm, at 2-cm intervals to a depth of 13 cm and subsequently at 4-cm intervals. Samples were prepared following the standard procedure used in the Palaeoenvironmental Research Unit, NUIG, for the preparation of minerogenic sediments (Molloy and O’Connell 1991). This includes inter alia the addition of a known number of Lycopodium spores to each sample to permit the estimation of pollen concentration, treatment with 60% hydrofluoric acid (HF) to remove silt and clay, and acetolysis. Fine particle debris was then removed by use of a 5-μm sieve in an ultra-sonic water bath. Glycerol was used as both a storage and mounting medium.
Pollen identification was carried out using a Leitz Diaplan microscope with routine counting carried out under a magnification of 500x. Every effort was made to identify all pollen to the lowest taxonomic unit consistent with reliable identification. The main identification keys used were Beug (2004) and Moore et al. (1991). Although a pollen sum in excess of 300 was aimed for, this could not practically be achieved due to the extremely low pollen concentration (average pollen concentration = 2.37 × 103 grains per cm3) and high amount of mineral material remaining in the samples (average pollen count = 165 grains per sample). Non-pollen palynomorphs (NPPs) which include algal remains, fungal spores, charcoal particles of ≥ 37 µm and Spherical Carbonaceous Particles (SCPs) were also counted. Pollen diagram and percentage and concentration calculations were generated using CountPol ver 3.3 (Feeser 2010).
Statistical analyses
Statistical analyses were performed on the dated portion of the core (26 samples; 0–25 cm), with chironomid and pollen data in the remainder of the core (26–33 cm) interpreted qualitatively to provide historical context. Ordination analyses were completed using Canoco version 5.04 (ter Braak and Šmilauer 2012) on square-root transformed chironomid percentage data for all common taxa, i.e. taxa present in at least two samples with a relative abundance of ≥ 2% in at least one sample (Quinlan and Smol 2001). Both principal component analysis (PCA; linear model) and detrended correspondence analysis (DCA; unimodal model) were performed on the chironomid percentage data. The linear-based ordination was chosen by assessing the gradient length (1.7 standard deviations (SD); linear model recommended) of the species composition along the first axis (Birks 1995).
Redundancy analysis (RDA) was used to determine important environmental controls on chironomid community composition. The statistical significance of seven variables (δ15N, δ13C, TN, TOC, OC:TN, LOI550 and LOI950) was assessed using Monte Carlo permutation tests (499 unrestricted permutations). Insignificant variables (p > 0.05) were dropped from further analysis. The remaining environmental variables were then forward-selected in order of decreasing variance and selection continued as long as each additional variable remained a statistically significant predictor (p ≤ 0.05) of chironomid assemblage data. P-value correction was performed using selected false discovery rate, Holm’s correction and the Bonferroni correction, which were each tested separately prior to model acceptance. Eigenvalue ratios (λ1/λ2) and partial RDAs were performed for the forward-selected variables to further identify the dominant environmental controls.
The role of temperature in chironomid compositional change was tested by running a similar forward-selected RDA for the top 13 samples (to coincide with available regional temperature data, see below) with temperature included as an additional environmental variable. Temperature data were adjusted to match the resolution of the lake sediment samples. As each 1-cm section of lake sediment represented between 2 and 6 years (following the 210Pb CRS model), air temperature data were averaged over each 1-cm sediment interval to correspond with the samples. After testing the correlations of monthly and seasonal data with chironomid PCA Axis 1, July temperature showed the strongest relationship and was used in ordination analysis. Although temperature was not forward-selected in the analysis conform to journal format requirements, the strength of the relationship was further explored through partial RDAs with the forward-selected variables.
Regional data
Open source environmental data were used for comparison with palaeolimnological findings from Lake Bander. Annual temperature from 1960 to 2018 for the region was sourced from Tuotuohe River Meteorological Monitoring Station (34° 13′ N, 92° 26′ E). Annual average nitrogen and compound fertiliser application rates from 1987 to 2016 for the Qinghai Province were obtained from National Bureau of Statistics of China. Nitrate (NO3) concentration (ppm) data from the Himalayan Dasuopu ice core were used as a proxy for the regional atmospheric nitrogen; dust concentration of 0.63–20 um diameter (per ml) from the same ice core record was also utilised, which spans from 1910 to 1996 (Thompson et al. 2000). Natural and anthropogenic sources of ammonia (NH4), nitrate (NO3) and total nitrogen (TN) deposition for 2015 were modelled for the study area using methods outlined in Qiao et al. (2019), which was originally based on the open source atmospheric science and air quality database, Community Multiscale Air Quality Modeling System (CMAQ) version 5.0.1.
Results
Dating model and sedimentation rate
Multi-proxy analyses were carried out on the datable portion of the core (0–25 cm) over the time interval 1909–2014, with a temporal resolution of 2–8 yr cm−1 (median = 4 yr cm−1). The sedimentation rate for BL-B is low at the beginning of the record with an average of 0.2 cm yr−1 from 33 to 22 cm (pre-1909–1929), increasing to an average of 0.3 cm yr−1 from 21 to 4 cm (1935–1989). The sedimentation rate decreases abruptly after 4 cm, with an average of 0.2 cm yr−1 for the remainder of the record (Fig. 2).
Chironomid assemblage zones
A total of 26 chironomid taxa were identified, with 7 to 14 taxa per sample. Two statistically significant chironomid assemblage zones (CAZ) were determined using chironomid percentage data (Fig. 3).
CAZ 1 (33–6 cm; pre-1910–1984)
The chironomid composition in CAZ 1 was dominated by Paratanytarsus undifferentiated (undif.) with an average of 47%, followed by Chironomus anthracinus-type (~ 17%), Orthocladius oliveri-type (~ 8%) and Corynocera ambigua-type (~ 7%). Limnophyes/Paralimnophyes (~ 6%) and Tanytarsus undif. (~ 4%) are also present in CAZ 1. Other taxa present in low abundances included Corynocera oliveri-type (average 2%), Psectrocladius sordidellus-type (~ 2%), Cricotopus intersectus-type (~ 2%) and Cricotopus shilovae-type (~ 2%).
CAZ 2 (5–0 cm; 1987–2014)
From the previous CAZ, Chironomus anthracinus-type increased overall to an average of ~ 25% including a sharp increase in abundance to 41% at 5 cm (1984). Increased abundance and frequency of Psectrocladius barbimanus-type is evident in CAZ 2, particularly at 0 cm depth (2014). Cryptochironomus showed a substantial increase in abundance from 0 to 6% in the top-most sample (0 cm depth; 2014). Cricotopus shilovae-type increased to an average of 5% during CAZ 2 including a sharp increase to 9% at 2 cm (2002). Small increases in percentages of Cricotopus intersectus-type (~ 2.5%) were also evident. Psectrocladius sordidellus-type decreased in abundance in CAZ 2 and is absent from 4 cm (1996) for the remainder of the record.
Geochemistry
Geochemistry results are presented in Fig. 4. Overall, the top 6 cm of the core (since 1984) showed nutrient influx and a general shift to more productive lake conditions. δ15N values remained steady from 25 to 22 cm (1909–1929), followed by a general decreasing trend to 3.8‰ at the top of the core. δ13C and OC:TN showed the highest values in the middle of the record, then generally decreased from 6 to 0 cm (1984–2014). OC:TN average at 9 from 0–2 cm at the top of the core (2002–2014). TN and TOC values remained relatively low through most of the record but increased steadily from 6 to 0 cm (1984–2014). Lastly, LOI550 increased gradually through the record, while LOI950 values showed a general decreasing trend from 25 to 6 cm (1909–1984), followed by increasing trend towards the top of the core (5–0 cm; 1987–2014).
Pollen assemblage zones
Pollen data for Lake Bander are presented in a percentage pollen diagram (Fig. 5). As per normal practice, percentage values are based on total terrestrial pollen (TTP) with the pollen sum (PS) excluding taxa of purely local origin, i.e. lake taxa and NPPs. On the basis of major shifts in the overall pollen representation, the pollen diagram was divided into three pollen assemblage zones (PAZ), the main features of which are outlined below.
Percentage pollen diagram plotted by depth with secondary axis in years CE. Ecological groups are differentiated using colour coding. Diagram includes from left to right, a composite percentage pollen diagram and selected pollen percentage curves. Scales are indicated at the base of the individual curves, with shaded curves presented at 1 × and open unshaded curves at 5 × and 10 × exaggeration for display purposes. Dots indicate very low values. The following abbreviations are used for rare occurrences: C = Carpinus; E = Ephedra; U = Utricularia. Chironomid assemblage zones (CAZ) are also included for ease of interpretation
PAZ 1 (33–17 cm; pre-1910–1951)
Non-arboreal pollen were the main contributors to the PS in PAZ 1. These included herbs (~ 54% TTP) and wetland/lake edge taxa (~ 42% TTP). The latter was accounted for primarily by Cyperaceae whereas many pollen types contribute to the herb representation in particular Artemisia and Poaceae with lesser amounts of Amaranthaceae which includes Chenopodiodeae, Thalictrum and Tubuliflorae subdivision of the Asteraceae. Algae (mainly Pediastrum but with significant levels of Botryococcus) were at the lowest representation for the profile. Arboreal pollen (AP) representation values average 3.5% with Pinus, Corylus, Alnus and Quercus the main contributors.
PAZ 2 (13–4 cm; 1958–1989)
Arboreal representation was higher in this PAZ with an average value of 5%. This was accounted for predominantly with an increase in the representation of Pinus, Picea and to a lesser extent Quercus. The percentage representation of herbs remained at ~ 55%, predominately accounted for by Poaceae and Artemisia and to a lesser extent Thalictrum, Tubuliflorae (mainly Bidens and Serratula types) and Amaranthaceae. Nymphaea was recorded from 7 cm (1982) but suberised basal hair cells of Nymphaeaceae were recorded earlier at 11 cm and subsequently at 5 cm (1966 and 1987, respectively). Overall algae had an increased representation for this PAZ with Pediastrum achieving > 70% representation at 9 and 7 cm (1975 and 1982, respectively). Charcoal particles reached a profile high of 43% at 9 cm (1975).
PAZ 3 (3–0 cm; 1996–2014)
Overall arboreal pollen representation was substantially higher in this upper PAZ. This reflects increased representation of Pinus and in particular Corylus which achieves 35% representation in the upper sample. Betula and Alnus were also more consistently recorded in this PAZ. Herbs while still at high representation showed an overall decline towards the top of the core. Pediastrum representation was high overall with values in excess of 70% for three of the four samples in this PAZ. SCPs were at low levels in the lower part of this PAZ and were not recorded from the upper two samples.
Statistical analysis
PCA Axis 1 (\(\lambda \) = 0.297) values highlight a change in the chironomid community composition in CAZ 2 of the stratigraphy (Figs. 3,6a). A PCA tri-plot is displayed in Fig. 6a, with environmental variables (δ15N, δ13C, TN, TOC, OC:TN, LOI550 and LOI950) plotted passively over the chironomid data. Results highlight a relationship of CAZ 2 samples and select taxa (Psectrocladius barbimanus-type, Cryptochironomus and Cricotopus shilovae-type) with high values of TN. The PCA tri-plot also indicates an association between Psectrocladius sordidellus-type and high values of OC:TN and δ13C.
A Principal component analysis (PCA) tri-plot for chironomid samples and species with environmental variables passively plotted; highlighting changes (samples) and similarities (species) in species composition with associated variables through time. B Redundancy analysis (RDA) of chironomid samples and species tri-plots with forward-selected environmental variables for Lake Bander. Symbols are used to denote chironomid-derived zonation for Lake Bander. Chironomid taxa abbreviations as follows: Chironomus anthracinus-type = Ch.ant, Corynocera ambigua = C.amb, Corynocera oliveri-type = C.oli, Corynoneura-arctica-type = Co.arc, Cricotopus intersectus-type = Cr.int, Cricotopus shilovae = Cr.sh, Cryptochironomus = Crypt, Eukiefferiella claripennis-type = Euk.clr, Hydrobaenus = Hydro, Limnophyes/Paralimnophyes = Limn, Orthocladius consobrinus-type = O.con, Orthocladius oliveri-type = O.oli, Paratanytarsus undifferentiated = Parat, Psectrocladius barbimanus-type = Psect.b, Psectrocladius sordidellus-type = Psect.s, Tanytarsus undifferentiated = Tanyt, Trissocladius = Triss
RDA for Lake Bander produced eigenvalues of 0.262 for Axis 1 and 0.052 for Axis 2, using two statistically significant forward-selected variables (δ15N and TN) (Fig. 6b and Table 1). TN exhibited the strongest relationship to RDA Axis 1, followed by δ15N. Similar to the PCA results, the combined species and sample tri-plot highlights a general shift in the chironomid community composition in CAZ 2, with Psectrocladius barbimanus-type, Cryptochironomus and Cricotopus shilovae-type exhibiting a strong relationship with increased TN. Partial RDAs indicate that TN accounts for a significant proportion of the variance in the chironomid community composition, explaining 22.2% of the variance on its own and 16.9% with δ15N partialled out (Table 1).
RDA for the top 13 samples, which included July temperature as an environmental variable, only had one forward-selected variable (TN), as no other variables remained significant after TN was included in the model (Fig. 7). The top three samples (0–3 cm; 2014–2002), which occur after a step-change in July temperature (Fig. 8), show a significant shift in chironomid assemblages since the late 1990s. Although temperature is also a significant predictor of chironomid change (adj. variance 26.1%, Fig. 7b), it does not remain a significant predictor once TN is included as a co-variable. However, TN remains a significant predictor (adj. variance 9.8%, Fig. 7b) over-and-above the effects of July temperature.
A Chironomid RDA tri-plot with forward-selected environmental variable (TN) for top 13 samples to coincide with the available temperature record (Tuotuohe River, 1960–2019). B Partial RDAs for TN (as the only forward-selected variable) and July temperature are included to explore the influence of temperature change since 1960. Symbology and chironomid abbreviations as in Fig. 6. Years indicate midpoint of sample
Comparison of TN and PCA Axis 1 values from Lake Bander with environmental data (average July temperature (Tuotuohe River Meteorological Monitoring Station), compound and nitrogen fertiliser (National Bureau of Statistics of China) and nitrates (NO3) concentration (ppm) and dust concentration (per ml; 0.63–20 um diameter) data (Thompson et al. 2000)). Pie charts display sources of annual deposition of ammonia, nitrate and total nitrogen in the study area using data from the Community Multiscale Air Quality Modeling System. Legend: IC/BC = Initial Condition and Boundary Condition
Discussion
Our results show a clear change in the environmental conditions within Lake Bander post-1985, with a shift from mesotrophic to more eutrophic conditions based on the chironomid, NPP (mainly algal remains) and geochemical data. Other palaeoenvironmental records identify climate change and nutrient influx as dominant controls on lake systems in the region (Chen et al. 2018; Kang et al. 2019; Lami et al. 2010). However as outlined below, at Lake Bander, despite increasing temperatures in recent years, available evidence suggests that N-influx is the main driver behind chironomid community change.
Lake catchment pre-1985
The geochemical data (δ15N, δ13C, OC:TN) together with the pollen, NPP and chironomid autecology reflect a natural oligotrophic to mesotrophic lake state with minimal anthropogenic impact pre-1985, as evidenced by low TN values (average 0.2%) along with higher OC:TN (average 10.3) and δ13C values during this time. Typically, OC:TN values of > 10 indicate organic matter of terrestrial origin or accumulation in less productive lake conditions, while < 10 infer lacustrine origin (primarily phytoplankton) and more productive conditions within the lake system (Cohen 2003).
The pollen record reflects an open landscape dominated by alpine steppe/meadow-type vegetation, likely extending back into the nineteenth century. This vegetation type consists of herb-rich sedge and grass communities that include mugwort, daisies, dandelions, rue, buttercups and members of the goosefoot (Amaranthaceae) and chickweed (Caryophyllaceae) families. The pollen record is similar to modern pollen assemblages from the Qinghai-Tibetan Plateau (Lu et al. 2011). The increase in arboreal pollen, mainly Pinus, post 1958 may reflect long-distance transport rather than an increase in tree cover at this time. Similarly, the microscopic charcoal particles which are high throughout may arise from local fires although they might also reflect long-distance transport in this open landscape.
The autecology of chironomid taxa in the sediment record generally indicates a cold, shallow, mesotrophic lake system including the presence of macrophytes and aggregations of algae. The presence of Chironomus anthracinus-type and Psectrocladius sordidellus-type in the record like infer moderately productive lake conditions. Chironomus anthracinus-type, a taxon which mainly inhabits oligotrophic and mesotrophic waters, is intolerant of hypoxia and is frequently found to be a dominant taxon in large lake systems (Moller Pillot 2009; Vallenduuk et al. 1997). Psectrocladius sordidellus-type, is often recorded in phosphate-poor, well-oxygenated water and is common in less eutrophic conditions. P. sordidellus-type however, has also been found in hypertrophic or polluted lake systems (Moller Pillot 2013). In this context, the presence of C. anthracinus-type and P. sordidellus-type together with the lake sediment geochemistry and NPP record, likely indicate mesotrophic conditions at Lake Bander during this time. The abundances of Paratanytarsus undif., P. sordidellus-type, Limnophyes/Paralimnophyes and Cricotopus shilovae-type indicate the presence of macrophytes within the shallow lake system (Brooks et al. 2007; Laug et al. 2020; Moller Pillot 2013). Additionally, Cricotopus shilovae-type, which is found amongst aquatic vegetation including aggregations of algae, on higher aquatic vegetation and the biofouling layers on stones and silt with plant detritus (Plank 2010), has also been recorded alongside high abundances of C. anthracinus-type in other chironomid assemblages from the region (Laug et al. 2020). Cold stenotherms in this section of the core include Corynocera ambigua-type (also associated with shallow lakes), Orthocladius oliveri-type and Corynocera oliveri-type (associated with oligotrophic conditions) (Brooks et al. 2007; Plank 2010; Self et al. 2011). The PCA tri-plot highlights a relationship with the P. sordidellus-type and CAZ 1 samples with higher values of OC:TN and δ13C, likely inferring less productive lake conditions (Fig. 6).
By 1975, δ15N values start to decline coinciding with a change in the algal record. The increased representations of Pediastrum [plate-like microscopic green algae (Shubert and Gärtner 2015)] and Botryococcus [a widespread unicellular photosynthetic, colonial microalga (Banerjee et al. 2002)] likely infer catchment disturbance and changes in lake productivity. Pediastrum, in particular, has been previously linked to catchment disturbance leading to increased nutrient input (Chique et al. 2017) and subtypes of Pediastrum in Klamt et al. (2021) have been associated with anthropogenic disturbances in a large shallow lake in Southwest China. The timing of this disturbance is in keeping with the beginning of the modernised agricultural economy in the region.
Nutrient influx post-1985
Lake sediment geochemistry and regional open-source environmental data provide evidence of increased N-deposition post-1985. Low δ15N (average + 4‰) and increasing TN values towards the top of the core are suggestive of increased input of nitrogen from anthropogenic sources such as synthetic fertilisers and/or industrial sources of atmospherically deposited N at Lake Bander. Typically, atmospheric N-deposition (NOx—oxidised to HNO3—dissociated to NO3) from human activities such as fossil fuel combustion (i.e. vehicle emissions, energy generation and industry) and natural processes (i.e. biogenic soil emissions, biomass burning and lightning) reflect lower δ15N values in lake sediment, ranging from —15 to + 15 ‰ (Kendall et al. 2007). Atmospheric NO3 deposition is usually considered to be a 15 N-depleted end-member in lake sediments (Xu et al. 2016). Similarly, δ15N values associated with synthetic N-fertilisers are also generally low (Wolfe et al. 2001), with values ranging between — 4 and + 4 ‰ (Botrel et al. 2014). It was hypothesised that the increased numbers of bar-headed geese at Lake Bander may have contributed to lake eutrophication, however animal waste typically exhibits higher δ15N values (as high as > 20‰; Kendall 1998) and is thus not considered a major source of modern N-influx at this site.
The timing of the shift recorded in the sediments of Lake Bander coincides with the significant increase in the global and regional nitrogen budget as a result of population growth, food production, industrial development, vehicle emissions and the burning of fossil fuels (Fowler et al. 2013; Galloway 2013). China’s NOx emissions have almost tripled from 1980 to 2003 (Ohara et al. 2007). NO3 concentration (ppm) data from the Himalayan Dasuopu ice core (Thompson et al. 2000) shows an increasing trend in regional atmospheric NO3 deposition from the 1990s onwards in south-west China, together with increased dust concentration during this time (Fig. 8). The majority of the total annual nitrate deposition (TNO3; 62%) in Qinghai province is derived from the Initial Condition and Boundary Condition (IC/BC), i.e. mainly from foreign sources, presumably from the industrial sector outside of the region (Fig. 8). As the study area is strongly influenced by monsoonal circulation and lakes act naturally as a sink for atmospheric N-deposition, long-range transport of atmospheric NO3 from other regions is likely being deposited at Lake Bander through monsoon precipitation (Liu et al. 2015; Xu et al. 2016), contributing to a proportion of the N-deposition recorded at this site.
Agriculture is also responsible for a significant proportion of regional nitrogen pollution due to excessive production and use of synthetic fertilisers in recent decades. Fertilisers are used to increase crop yields, however only 27.5–35% of the N applied is taken up by plants (Yu et al. 2015), and the surplus is discharged into waterbodies or volatilized into the atmosphere (Chen et al. 2018). Nutrient excess (synthetic fertiliser use) from the rapid development of China’s agricultural economy from the 1980s (Li et al. 2012) including an increase of 45.3% from 2000 to 2015 (Pan et al. 2019), is likely a source of N at Lake Bander. Results from the locally applied CMAQ model show agriculture was responsible for 93% of ammonia (NH4) deposition in the study area in 2015 (Fig. 8). Overall, 72% of TN deposition was derived from agricultural sources in the region, with NH4 contributing a larger proportion of the TN deposition than NO3 from foreign sources in 2015 (Fig. 8). Annual average nitrogen and compound fertiliser application rates have increased significantly from 1987 to 2016 in Qinghai (Fig. 8; National Bureau of Statistics of China). Increased synthetic fertiliser application has been shown to result in airborne N-deposition on the Tibetan Plateau, as evident in the high-elevation, Dasuopu ice core record (Li et al. 2020b), as well as precipitation (Liu et al. 2015) and plant (Zong et al. 2016) sampling.
Additionally, atmospheric dust deposition can provide nutrient enrichment to remote unproductive lake systems. Anthropogenic and climatic-induced dust deposition has been shown to affect surface-water alkalinity, aquatic productivity and terrestrial nutrient cycling in remote lacustrine ecosystems (Neff et al. 2008). Thompson et al. (2000) related the increased atmospheric dustiness from the Himalayan Dasuopu ice core in modern times with a possible reduction in snow cover, increased aridity, and/or increased agricultural activity in the source regions (Fig. 8).
Ecological impact of nutrient influx
The magnitude of modern anthropogenic activity is evident at Lake Bander, despite its remote location and local protection measures through the designation of the Sanjiangyuan National Park and conservation programmes (e.g. Ecological Protection and Restoration Program (EPRP)). The lake system has been significantly impacted by increased nutrient input from N-deposition and possibly increased dust concentration post-1985. Nutrient input has influenced lake productivity in a shift to more eutrophic lake conditions. OC:TN decreases slightly to an average of 9 in modern samples (2002–2014), inferring more aquatic productivity within the lake system. As TOC and TN are positively correlated (Pearson’s correlation coefficient of 0.964), this generally indicates that sedimentary nitrogen is mostly in organic form (Carsten and Stephen 2001; Nara et al. 2014); therefore OC:TN can provide a good insight into the origin of organic matter (Sun et al. 2016). In lake systems with low allochthonous organic matter input, as in Lake Bander during this time, there are multiple factors which may control δ13C and δ15N values in a modern lacustrine environment, including early diagenesis which can potentially alter the isotopic signal as an environmental indicator (Meyers 1997; Sun et al. 2016). At Lake Bander, early diagenesis does not appear to be a strong factor, as δ13C and δ15N values would show a down-core decrease over time if early diagenesis was prominent (Sun et al. 2016).
Chironomid community composition also reflects a change in trophic conditions, as indicated by changes in the less dominant taxa, such as Psectrocladius barbimanus-type, P. sordidellus-type, Cricotopus shilovae-type, and Cryptochironomus, rather than large changes in the dominant taxa. Chironomids are indirectly affected by nutrient enrichment through altered microhabitats and food chains i.e. changes in substrate, macrophytes, increased lake algal biomass and fish conditions. The most notable change in the chironomid composition post-1985 is the sharp increase in P. barbimanus-type from 2002 and the absence of P. sordidellus-type from the record from 1989. P. barbimanus-type is a taxon common in water bodies with high conductivity, high pH and calcium content (Moller Pillot 2013). P. barbimanus-type has also been found in abundance in sand filter beds with decomposed organic matter and sometimes in hypertrophic conditions (Moller Pillot 2013; Steenbergen 1993). Increased photosynthesis associated with eutrophication can deplete dissolved inorganic carbon and increase the alkalinity of the lake water (Chislock et al. 2013). Additionally, P. barbimanus-type is commonly found in water bodies with high calcium content. Calcium content was likely produced from increased biogenic sources as a result of increased productivity or changes in the macrophytes community. P. barbimanus-type also reflects a strong association with TN in all ordinations (Figs. 6 and 7).
Cryptochironomus is associated with nutrient-rich waters (Brooks et al. 2007), although it can also be found in less polluted waters with high oxygen content (Moller Pillot 2009). The ‘surface-dwelling’ species, i.e. inhabiting the top centimetre of lake water, has adapted physiological and behavioural changes in response to low dissolved oxygen content associated with eutrophication (Brodersen and Quinlan 2006). Cryptochironomus has the ability to ventilate oxygen-rich water and can also migrate to shallower sections of the lake when bottom depths become hypoxic and re-colonise the original area once again with improved oxygen conditions (Bazzanti et al. 1998; Brodersen and Quinlan 2006). Cryptochironomus shows an abrupt increase in abundance in 2014, although one sample of 6% may seem insignificant, this taxon is recorded in very low densities usually less than 100 larvae per m2 in comparison to C. anthracinus-type which is recorded in densities of about 1000 larvae per m2 (Moller Pillot 2009, 2013), therefore the recording is considered noteworthy. The abrupt increase in abundance is likely due to increased food availability associated with nutrient enrichment, as Crypotochironomus mainly feed on Oligochaeta, small Chironomidae and Crustacea (Moller Pillot 2009; Vallenduuk and Morozova 2005).
The overall change in composition is clearly visible in the PCA tri-plot, particularly in the modern samples from 2002 to 2014, displayed to the extreme right of the tri-plots (Figs. 3, 6a, 7). Furthermore, several taxa which inhabit algae and higher aquatic vegetation (P. barbimanus-type and Cricotopus shilovae-type) and feed on algae blooms (Cricotopus intersectus-type) show an increased abundance from 2002, coinciding with increased representation of Nymphaea, Nymphaeaceae basal hair cells, Pediastrum and Botryococcus in the pollen and NPP records and a decrease in OC:TN values. The increased nutrient enrichment likely stimulated algae production, with increased representation of Pediastrum in the top-most samples. The decreasing δ13C values are likely to be a result of the increased Pediastrum abundances, as δ13C values from lacustrine algae are typically low, ranging between —30 and —25‰ (Meyers 2003). RDA and partial RDAs show a strong association between the chironomid samples from CAZ 2 with increased TN. As previously mentioned, TN largely arises from atmospheric NO3 from extra-regional sources and NH4 from synthetic fertiliser application emissions at Lake Bander. As TN explains 22.2% of the variance on its own and 16.9% with other variables partialled out, TN appears to be the main driver behind chironomid compositional change at Lake Bander in recent decades.
Global warming has also been shown to contribute to higher lake productivity in the region (Lami et al. 2010). RDA for the top 13 samples (since 1960), which included July temperature as an environmental variable, did not identify July temperature as a forward-selected variable. Rather, TN was the only forward-selected variable and remained the strongest control on chironomid community change during this time. TN remained a significant predictor even after July temperature was included in the RDA as a co-variable. However, temperature does not remain a significant predictor of chironomid community change after TN is included as a co-variable. Additionally, the statistical zonation of the chironomid data infers a shift in the community composition in 1985, when PCA Axis 1 scores, TN and regional fertiliser application all begin to increase. If climate was the dominant factor behind community change, the division would have occurred during the late 1990s when July temperatures exhibit a step increase in the region.
Although temperature does not show a strong statistical link with the chironomid community at Lake Bander, the top three samples, which occur after the step-change in temperature in the late 1990s, do show a noticeable change from the samples below (Fig. 7). This is largely due to the emergence of P. barbimanus-type and Cryptochironomus and the disappearance of cold-stenotherms C. oliveri-type and O. oliveri-type from the record. So, although significant changes in the lake began before major warming from the late 1990s and were likely not initiated by temperature, warming of the past two decades may be reinforcing this change and pushing the lake further towards eutrophic conditions (Moss et al. 2011).
In terms of local changes to vegetation cover the pollen record suggests some expansion of scrub/woodland into the alpine grasslands from 2002 onwards. However, as there is no evidence of woodland encroachment in the immediate vicinity, the record from Lake Bander likely reflects long-distance airborne transport of arboreal pollen which has been shown to disperse over long distances in this region (Li et al. 2020a). The rise in arboreal pollen may for instance reflect the observed expansion of shrublands and forest recorded in parts of the southeastern Qinghai-Tibetan Plateau between 2001 and 2015, thought to be a response to warming and/or the implementation of conservation policies (Chao et al. 2019). Finally, the abrupt drop in sedimentation rate and concomitant increase in LOI from 1996 most likely reflects soil stabilisation with less activity and/or erosion in the catchment, which could be a result of localised conservation measures (Li et al. 2012).
Conclusion
This multi-proxy palaeoenvironmental reconstruction provides an important record of land-use change and the ecological impacts of N-deposition in Lake Bander, a remote lake within the TRHR. Chironomid, pollen and NPP analyses coupled with lake sediment geochemistry reflect a mesotrophic lake system for the majority of the twentieth century with minimal anthropogenic impact. A shift in the lake environment to eutrophic conditions is evident from 1985 with an intensification of nutrient enrichment most likely arising from airborne N-deposition from increased use of synthetic fertilisers and from extra-regional industrial sources. Although temperature was not identified as a primary driver of eutrophication recorded at Lake Bander, anthropogenic warming likely exacerbated the eutrophication process in recent decades. This study highlights the far-reaching repercussions of modern agricultural and industrial activity on the Qinghai-Tibetan Plateau and demonstrates the benefit of palaeolimnological reconstruction techniques in ecosystem monitoring programmes.
References
Andersen T, Cranston PS, Epler JH (2013) The larvae of Chironomidae (Diptera) of the Holarctic region–Keys and diagnoses. Insect Syst Evol 66:550
Banerjee A, Sharma R, Chisti Y, Banerjee UC (2002) Botryococcus braunii: a renewable source of hydrocarbons and other chemicals. Crit Rev Biotechnol 22:245–279
Bazzanti M, Seminara M, Baldoni S, Dowgiallo MG (1998) Assessing hypolimnetic stress in a monomictic, eutrophic lake using profundal sediment and macrobenthic characteristics. J Freshw Ecol 13:405–412
Bennett KD (2009–1993). psimpoll. Version 4.27. Queen’s University Belfast, Belfast
Beug HJ (2004) Leitfaden der Pollenbestimmung für Mitteleuropa und angrenzende Gebiete. Pfeil, München
BirdLife International. http://www.birdlife.org/, accessed on the 03/07/2020.
Birks HJB (1995) Quantitative paleoenvironmental reconstructions. In: Maddy D, Brew JS (eds) Statistical modelling of quaternary science data. Quaternary Research Association, London, pp 161–254
Blaauw M, Christen JA (2011) Bacon software for flexible Bayesian age-depth modelling. Queen’s University Belfast, Belfast
Botrel M, Gregory-Eaves I, Maranger R (2014) Defining drivers of nitrogen stable isotopes (δ15N) of surface sediments in temperate lakes. J Paleolimnol 52:419–433
Brodersen KP, Quinlan R (2006) Midges as palaeoindicators of lake productivity, eutrophication and hypolimnetic oxygen. Quat Sci Rev 25(15–16):1995–2012
Brooks SJ, Langdon PG, Heiri O (2007) The identification and use of palaearctic chironomidae larvae in palaeoecology. Quaternary Research Association, London
Carpenter SR (2005) Eutrophication of aquatic ecosystems: bistability and soil phosphorus. Proc Natl Acad Sci USA 102:10002–10005
Carpenter SR, Caraco NF, Correll DL, Howarth RW, Sharpley AN, Smith VH (1998) Nonpoint pollution of surface waters with phosphorus and nitrogen. Ecol Appl 8:559–568
Carsten JS, Stephen EC (2001) Nitrogen and carbon isotopic composition of marine and terrestrial organic matter in Arctic Ocean sediments: implications for nutrient utilization and organic matter composition. Deep-Sea Res I Oceanogr Res Pap 48:789–810
Chao W, Qiong G, Mei Y (2019) Quantifying trends of land change in Qinghai-Tibetan plateau during 2001–2015. Remote Sens 11:2435
Chen X, Wang Y, Ye C, Zhou W, Cai Z, Yang H, Han X (2018) Atmospheric nitrogen deposition associated with the eutrophication of Taihu Lake. J Chem 2018:1–10
Chique C, Molloy K, Potito AP (2017) Mid-late holocene vegetational history and land-use dynamics in county monaghan, northeastern ireland—the palynological record of lough Muckno. J N Atl 32:1–24
Chislock MF, Doster E, Zitomer RA, Wilson AE (2013) Eutrophication: causes, consequences, and controls in aquatic ecosystems. Nat Educ Know 4(4):10
Cohen AS (2003) Paleolimnology: the history and evolution of lake systems. Oxford University Press, Oxford
Du M, Kawashima S, Yonemura S, Zhang X, Chen S (2004) Mutual influence between human activities and climate change in the Tibetan Plateau during recent years. Glob Planet Change 41:241–249
EBird.org. https://ebird.org/checklist/S45164146. Accessed 03 July 2020
Eggermont H, Heiri O (2012) The chironomid-temperature relationship: expression in nature and palaeoenvironmental implications. Biol Rev 87:430–456
Fayiah M, Dong S, Khomera SW, Ur Rehman SA, Yang M, Xiao J (2020) Status and challenges of Qinghai-Tibet Plateau’s Grasslands: an analysis of causes, mitigation measures, and way forward. Sustainability 12:1099
Feeser I (2010) CountPol version 3.3. Palaeoenvironmental Research Unit, National University of Ireland Galway
Fowler D, Coyle M, Skiba U, Sutton MA, Cape JN, Reis S, Sheppard LJ, Jenkins A, Grizzetti B, Galloway JN, Vitousek P, Leach A, Bouwman AF, Butterbach-Bahl K, Dentener F, Stevenson D, Amann M, Voss M (2013) The global nitrogen cycle in the twenty-first century. Philos Trans R Soc B 368:20130164
Galloway JN, Leach AM, Bleeker A, Erisman JW (2013) A chronology of human understanding of the nitrogen cycle. Philos Trans R Soc B 368:20130120
Green-River.org. Let me fly higher—Band-headed Goose Guardianship and Investigation (2012–2016). http://www.green-river.org/show-12-11-1.html. Accessed 17 June 2020
Guo X, Potito AP, Luo L, Beilman DW (2013) Twentieth century human and climate impacts on a large mountain lake in southwest China. Hydrobiologia 718:189–206
Harris D, Horwáth WR, van Kessel C (2001) Acid fumigation of soils to remove carbonates prior to total organic carbon or CARBON-13 isotopic analysis. Soil Sci Soc Am J 65:1853–1856
Harris IC, Jones PD, Osborn T (2020) CRU TS4.04: climatic research unit (CRU) time-series (TS) version 4.04 of high-resolution gridded data of month-by-month variation in climate (Jan. 1901–Dec. 2019). Centre for Environmental Data Analysis, https://catalogue.ceda.ac.uk/uuid/89e1e34ec3554dc98594a5732622bce9. Accessed 08 June 2020
Heiri O, Lotter AF, Lemcke G (2001) Loss on ignition as a method for estimating organic and carbonate content in sediments: reproducibility and comparability of results. J Paleolimnol 25:101–110
Hu Z, Anderson NJ, Yang X, McGowan S (2014) Catchment-mediated atmospheric nitrogen deposition drives ecological change in two alpine lakes in SE Tibet. Glob Change Biol 20:1614–1628
Jeppesen E, Moss B, Bennion H, Carvalho L, De Meester L, Feuchtmayr H, Friberg N, Gessner MO, Heffting M, Lauridsen T, Liboriussen L, Malmquist HJ, May L, Meerhoff M, Olafsson JS, Soons MM, Verhoeven JTA (2010) Interaction of climate change and eutrophication. In: Kernan M, Battarbee RW, Moss B (eds) Climate change impacts on freshwater ecosystems. Blackwell Publishing, Sussex, pp 119–151
Jiang L, Yao Z, Liu Z, Wang R, Wu S (2015) Hydrochemistry and its controlling factors of rivers in the source region of the Yangtze River on the Tibetan Plateau. J Geochem Explor 155:76–83
Juggins S (2016) Program C2 data analysis. Version 1.7.7. University of Newcastle, Newcastle
Kang W, Chen G, Wang J, Huang L, Wang LR, Hu K, Liu Y, Tao J, Blais JM, Smol JP (2019) Assessing the impact of long-term changes in climate and atmospheric deposition on a shallow alpine lake from southeast Tibet. Sci Total Environ 650:713–724
Kendall C (1998) Tracing nitrogen sources and cycling in catchments. In: Kendall C, McDonnell JJ (eds) Isotope tracers in catchment hydrology. Elsevier, Amsterdam, pp 519–576
Kendall C, Elliott EM, Wankel SD (2007) Chapter 12 Tracing anthropogenic inputs of nitrogen to ecosystems. In: Michener R, Lajtha K (eds) Stable isotopes in ecology and environmental science. Blackwell Publishing Ltd, Second Edition, pp 375–449
Klamt AM, Qian F, Hu K, Wang J, Huang L, Li R, Chen G (2021) The rise and fall of primary producers and consumers in a multiply-stressed shallow lake (Lake Qilu, China) over the last 200 years. Eco Indic 129:107891
Lami A, Turner S, Musazzi S, Gerli S, Guilizzoni P, Rose NL, Yang H, Wu G, Yang R (2010) Sedimentary evidence for recent increases in production in Tibetan plateau lakes. Hydrobiologia 648:175–187
Laug A, Turner F, Engels S, Wang J, Haberzettl T, Ju J, Yu S, Kou Q, Börner N, Schwalb A (2020) Is there a common threshold to subfossil chironomid assemblages at 16 m water depth? evidence from the Tibetan Plateau. J Limnol 79(3):278–292
Li J, Xie G, Yang J, Ferguson DK, Liu X, Liu H, Wang Y (2020a) Asian Summer Monsoon changes the pollen flow on the Tibetan Plateau. Earth Sci Rev 202:103114
Li XL, Brierley G, Shi DJ, Xie YL, Sun HQ (2012) Ecological protection and restoration in Sanjiangyuan Natural Reserve, Qinghai Province, China. In: Higgitt D (ed) Perspectives on environmental management and technology in Asian River Basins. Springer, Dordrecht, pp 93–119
Li Z, Hastings MG, Wendell WW, Tian L, Clemens SC, Song L, Shao L, Fang Y (2020b) Isotopic evidence that recent agriculture overprints climate variability in nitrogen deposition to the Tibetan Plateau. Env Int 138:105614
Liao J, Shen G, Li Y (2013) Lake variations in response to climate change in the Tibetan Plateau in the past 40 years. Int J Digit Earth 6(6):534549
Liu X, Chen B (2000) Climatic warming in the Tibetan plateau during recent decades. International Int J Climatol 20:1729–1742. https://doi.org/10.1002/1097-0088(20001130)20:14<1729::AID-JOC556>3.0.CO;2-Y
Liu YW, Xu R, Wang YS, Pan YP, Piao SL (2015) Wet deposition of atmospheric inorganic nitrogen at five remote stations on the Tibetan Plateau. Atmos Chem Phys 15:11683–11700
Lu H, Wu N, Liu K, Zhu L, Yang X, Yao T, Wang L, Li Q, Liu X, Shen C, Li X, Tong G, Jiang H (2011) Modern pollen distributions in Qinghai-Tibetan Plateau and the development of transfer functions for reconstructing Holocene environmental changes. Quat Sci Rev 30:947–966
Luo C, Wang S, Zhao L, Xu S, Xu B, Zhang Z, Yao B, Zhao X (2017) Effects of land use and nitrogen fertilizer on ecosystem respiration in alpine meadow on the Tibetan Plateau. J Soils Sediments 17:1626–1634
Meyers PA (1997) Organic geochemical proxies of paleoceanographic, paleolimnologic, and paleoclimatic processes. Org Geochem 27:213–250
Meyers PA (2003) Applications of organic geochemistry of paleolimnological reconstructions: a summary of examples from the Laurentian Great Lakes. Org Geochem 34:261–289
Moller Pillot HKM (2009) Chironomidae larvae: biology and ecology of the chironomini. KNNV Publishing, Zeist
Moller Pillot HKM (2013) Chironomidae larvae: biology and ecology of the aquatic orthocladiini. KNNV Publishing, Zeist
Molloy K, O’Connell M (1991) Palaeoecological investigations towards the reconstruction of woodland and land-use history at Lough Sheeauns, Connemara, western Ireland. Rev Palaeobot Palynol 67(1–2):75–113
Moore PD, Webb JA, Collinson ME (1991) Pollen analysis, 2nd edn. Blackwell Scientific, London
Moss B, Kosten S, Meerhoff M, Battarbee RW, Jeppesen E, Mazzeo N, Havens K, Lacerot G, Liu Z, De Meester L, Paerl H, Scheffer M (2011) Allied attack: climate change and eutrophication. Inland Waters 1(2):101–105
Nara FW, Watanabe T, Kakegawa T, Minoura K, Imai A, Fagel N, Horiuchi K, Nakamura T, Kawai T (2014) Biological nitrate utilization in south Siberian lakes (Baikal and Hovsgol) during the Last Glacial period: the influence of climate change on primary productivity. Quat Sci Rev 90:69–79
National Bureau of Statistics of China. http://www.stats.gov.cn/
Neff JC, Ballantyne AP, Farmer GL, Mahowald NM, Conroy JL, Landry CC, Overpeck JT, Painter TH, Lawrence CR, Reynolds RL (2008) Increasing eolian dust deposition in the western United States linked to human activity. Nat Geosci 1:189–195
Ohara T, Akimoto H, Kurokawa J, Horii N, Yamaji K, Yan X, Hayasaka T (2007) An Asian emission inventory of anthropogenic emission sources for the period 1980–2020. Atmos Chem Phys 7(16):6843–6902
Pan XD, Li P, Fang ZZ, Duan CQ (2019) Spatial and temporal variations in fertilizer use across prefecture-level cities in China from 2000 to 2015. Environ Sci 40(10):4733–4742
Plank A (2010) Chironomid-based inference models for Tibetan lakes aided by a newly developed chironomid identification key. PhD Thesis, Freie Universität Berlin
Qian Y, Sun F, Li B, Li S, Zhao J (2014) Early Permian-Late Triassic magmatism in the Tuotuohe region of the Qinghai-Tibet Plateau: constrains on the tectonic evolution of the western segment of the Jinshajiang Suture. Acta Geol Sin 88(2):498–516
Qiao X, Guo H, Tang Y, Wang P, Deng W, Zhao X, Hu J, Ying Q, Zhang H (2019) Local and regional contributions to fine particulate matter in the 18 cities of Sichuan Basin, southwestern China. Atmos Chem Phys 19:5791–5803
Qu B, Zhang Y, Kang S, Sillanpää M (2019) Water quality in the Tibetan Plateau: major ions and trace elements in rivers of the “Water Tower of Asia.” Sci Total Environ 649:571–581
Quinlan R, Smol JP (2001) Setting minimum head capsule abundance and taxa deletion criteria in chironomid-based inference models. J Paleolimnol 26:327–342
Rieradevall M, Brooks SJ (2001) An identification guide to subfossil Tanypodinae larvae (Insecta: Diptera: Chironomidae) based on cephalic setation. J Paleolimnol 25:81–99
Self AE, Brooks SJ, Birks HJB, Nazarova L, Porinchu D, Odland A, Yang H, Jones VJ (2011) The distribution and abundance of chironomids in high-latitude Eurasian lakes with respect to temperature and continentality: development and application of new chironomid-based climate-inference models in northern Russia. Quat Sci Rev 30(9–10):1122–1141
Shubert E, Gärtner G (2015) Nonmotile coccoid and colonial green Algae. Aquat Ecol 2:315–373
Steenbergen HA (1993) Macrofauna-atlas of North Holland: Distribution Maps and Responses to Environmental Factors of Aquatic Invertebrates, Haarlem
Sun W, Zhang E, Jones RT, Liu E, Shen J (2016) Biogeochemical processes and response to climate change recorded in the isotopes of lacustrine organic matter, southeastern Qinghai-Tibetan Plateau, China. Palaeogeogr Palaeoclimatol Palaeoecol 453:93–100
Taylor KJ, McGinley S, Potito AP, Molloy K, Beilman DW (2018) A mid to late Holocene chironomid-inferred temperature record from northwest Ireland. Palaeogeogr Palaeoclimatol Palaeoecol 505:274–286
Ter Braak CJF, Šmilauer P (2012) Canoco Reference Manual and User's Guide: Software for Ordination, Version 5.0. Microcomputer Power, Ithaca, USA
Thompson LG, Yao T, Mosley-Thompson E, Davis ME, Henderson KA, Lin PN (2000) A high-resolution millennial record of the South Asian Monsoon from Himalayan ice cores. Science 289:1916–1919
Vallenduuk HJ, Moller-Pillot HKM, van der Velde JA Wiersma SM, bij de Vaate A (1997) Bijdrag tot de kennis der Nederlandse Chironomidae (vedermuggen): de larven van het genus Chironomus. Dutch. RIZA rapport 97.053. Rijksinstituut voor Integraal Zoet- waterbeheer & Afvalwaterbehandeling, Lelystad
Vallenduuk HJ, Morozova E (2005) Cryptochironomus. An identification key to the larvae and pupal exuviae in Europe. Lauterbornia 55:1–22
Walker IR (2001) Midges: chironomidae and related diptera. In: Smol JP, Birks HJB, Last WM (eds) Tracking environmental change using lake sediments, vol 4. Zoological Indicators. Kluwer Academic Publishers, Dordrecht, pp 43–59
Wan D, Jin Z, Zhang F, Song L, Yang J (2016) Increasing dust fluxes on the northeastern tibetan plateau linked with the little ice age and recent human activity since the 1950s. Aeolian Res 23:93–102
Wolfe AP, Baron JS, Cornett RJ (2001) Anthropogenic nitrogen deposition induces rapid ecological changes in alpine lakes of the Colorado Front Range (USA). J Paleolimnol 25:1–7
Wu G, Zhang C, Xu B, Mao R, Joswiak D, Wang N, Yao T (2013) Atmospheric dust from a shallow ice core from Tanggula: implications for drought in the central Tibetan Plateau over the past 155 years. Quat Sci Rev 59:57–66
Wu Y, Wang S, Ni Z, Li H, May L, Pu J (2021) Emerging water pollution in the world’s least disturbed lakes on Qinghai-Tibetan Plateau. Environ Pollut 272:116032
Xu H, Yu K, Lan J, Sheng E, Liu B, Ye Y, Hong B, Wu H, Zhou K, Yeager KM (2016) Different responses of sedimentary d15N to climatic changes and anthropogenic impacts in lakes across the Eastern margin of the Tibetan Plateau. J Asian Earth Sci 123:111–118
Yu F, Shi W (2015) Nitrogen use efficiencies of major grain crops in china in recent 10 years. Acta Pedol Sin 52:1311–1324
Zelentsov NI (1989) A new species of Orthocladiinae of the genus Cricotopus v.d. Wulp and metamorphosis of Acricotopus longipalpus Reiss (Diptera, Chironomidae) from the Pamir. Trudy Instituta Biologii Vnutrennikh Vod 56:215–249
Zhang J, Zhang L, Liu W, Qi Y, Wo X (2014) Livestock-carrying capacity and overgrazing status of alpine grassland in the Three-River Headwaters region, China. J Geogr Sci 24(2):303–312
Zhang Y, Luan S, Chen L, Shao M (2011) Estimating the volatilization of ammonia from synthetic nitrogenous fertilizers used in China. J Environ Manag 92:480–493
Zhou K, Wu J, Liu H (2021) Spatio-temporal estimation of the anthropogenic environmental stress intensity in the Three-River-Source National Park region, China. J Clean Prod 318:128476
Zong N, Shi P, Song M, Zhang X, Jiang J, Chai X (2016) Nitrogen critical loads for an alpine meadow ecosystem on the Tibetan Plateau. Environ Manag 57:531–542
Acknowledgements
The study was supported by the International Collaboration Program of Sichuan Department of Science and Technology (2020YFH0023).
Funding
Open Access funding provided by the IReL Consortium. The study was supported by the International Collaboration Program of Sichuan Department of Science and Technology (2020YFH0023).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no conflict of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Luo, Y., Taylor, K.J., Potito, A.P. et al. Ecological impacts of N-deposition in a remote, high-elevation lake in the Three River Headwaters Region, Qinghai-Tibetan Plateau. J Paleolimnol 69, 141–160 (2023). https://doi.org/10.1007/s10933-022-00266-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10933-022-00266-5