Abstract
Polysorbates are non-ionic amphiphilic organic compounds, widely used as surfactants. They have a molecular weight in the 1200–1400 g mol−1 range, so they are on the borderline between monomeric and polymeric plasticizers. Therefore, they can potentially provide the benefits of both plasticizer types. In this work, polyethylene glycol sorbitan monolaurate (Tween® 20), and polyethylene glycol monooleate (Tween® 80) are proposed as environmentally friendly plasticizers for PLA with enhanced ductile properties. The addition of 20 wt% of polysorbates into a PLA matrix, leads to a noticeable increase in elongation at break, from 4.0% (neat PLA) up to values around 180%. The plasticization efficiency was assessed by the decrease in the glass transition temperature (Tg), from 61.0 ºC (neat PLA) down to such los values of 29.5 ºC, and 36.6 ºC, for plasticized PLA formulations with 30 wt% Tween® 20, and 30 wt% Tween® 80, respectively. Moreover, due to the high molecular weight of polysorbates, they are not highly volatile, which allows processing PLA by conventional extrusion and injection molding without plasticizer volatilization. This works widens the industrial applications of polysorbates, as cost-effective, highly efficient and environmentally friendly plasticizers for PLA with enhanced toughness.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
With the increasing environmental concern, biopolymers have raised as a need to replace or reduce petroleum-based polymers. Biopolymers can be classified attending to their origin and potential of biodegradation (or compostability). The best combination from an environmental standpoint is a group of polymers of natural origin which are also biodegradable. This group includes polysaccharides (starch, pectin, chitin, arabinoxylans, among others), proteins (gluten, soy protein, zein, pea protein, casein, among others), bacterial polyesters such as polyhydroxyalkanoates (PHA), and poly(lactide) (PLA) which can be obtained by ring opening polymerization (ROP) of lactide derived from starch fermentation. Despite this group of polymers is very attracting, their properties are still far from most of petroleum-based polymers in both performance and cost. Poly(lactide) (PLA) is, with difference, one of the most promising biopolymers and is commercially available at a reasonable cost and balanced properties which make it suitable for a wide range of applications, including packaging industry, medical sector, furniture, automotive industry, textiles, toys, 3d-printing material and so on [1].
Despite its relatively easy processing, good transparency, moderate barrier properties, its main drawback lies on its intrinsic brittleness due to a very low elongation at break, which leads to low toughness too [2, 3]. This fact limits its widespread use, and, in many cases, specific additives are required to overcome this. Copolymerization is a technical solution to overcome brittleness by inserting a flexible chain into the PLA backbone [4]. Different monomers have been proposed to improve PLA toughness such as ε-caprolactone [5], δ-valerolactone [6], glycolide [7], ethylene glycol [8], propylene carbonate [9], dibutyl maleate [10] and monobutyl maleate [11] which have been reported to provide increased flexibility. Nevertheless, control of the copolymerization to tailor the desired properties is complex (comonomers ratio, temperature, time, type and amount of catalysts), and consequently, this technology is not always transferred to an industrial scale [12]. Another technical approach is blending with flexible polymers such as poly(ε-caprolactone) (PCL), poly(butylene succinate) (PBS), poly(butylene succinate-co-adipate) (PBSA), poly(butylene adipate-co-terephthalate) (PBAT), poly(diethylene glycol succinate) [13, 14]. The final performance of these blends highly depends on miscibility. As most of the above-mentioned polymers show poor compatibility with PLA, the improvement in flexible properties is not usually remarkable. Moreover, blending PLA with immiscible polymers leads to the loss of transparency [15]. Finally, plasticization has been traditionally used to give improved toughness to PLA. Plasticizers usually promote a shift of the glass transition of PLA (Tg) to lower values, thus enhancing plastic deformation.
A wide range of monomeric (e.g. citrates, terpenes and terpenoids, cinnamic acid esters, itaconates, tartrates), and oligomeric/polymeric plasticizers (e.g. oligomers of lactic acid-OLA, polyethylene glycol-PEG, polypropylene glycol-PPG, polysebacates, polyadipates), have been proposed as plasticizers for PLA [16,17,18,19,20,21]. Low molecular weight monomeric plasticizers give exceptional ductile properties to PLA, but they offer higher migration than polymeric plasticizers. Phtalates such as dioctyl phthalate (DOP) or diisononyl phthalate (DINP) have been reported to be excellent plasticizers with a very low cost, however, due to their toxicity and migration problems, other environmentally friendly plasticizers are being investigated as alternatives [22, 23]. Also, PEG and PPG have always been reported as an excellent plasticizer for PLA, as they offer a very good elongation at break and a considerable reduction of the glass transition temperature [24], nonetheless, their petroleum-derived origin makes them decrease the environmental potential of PLA, and therefore, other alternatives are being explored. Polysorbates have been extensively used as non-ionic surfactants with applications in cleaners, personal care and cosmetics, pharmaceutical applications [25], and fragrance ingredients, as well as emulsifying agents. Merle et al. [26] reported the use of Tween® 80 as surfactant for the production of bio-based foams. However, their use as plasticizers for biobased injection-molded thermoplastic parts has not been extensively studied. Thauvin et al. [27] reported on the use of Tween® 60 and Tween® 65 in the development of PLA formulations for control drug delivery, developing polymers with great hydrophilicity. Their particular chemical structure is very interesting since they offer hydrophilic groups (ethylene oxide units), as well as hydrophobic groups (mainly fatty acids), and they have been proposed as plasticizers for PLA since their solubility parameters are very close. Polysorbate 20 (Tween® 20) and polysorbate 80 (Tween® 80) have a solubility parameter of 22.10 and 21.30 MPa1/2 as reported by Shakeel et al. [28], and the solubility parameter for PLA ranges from 20.7 to 21.7 MPa1/2 [29, 30] Moreover, polysorbates possess high molecular weight compared to other monomeric plasticizers and this feature could be of special interest to prevent plasticizer from migration [31]. Polysorbates have been proposed as plasticizers for poly(ethylene oxide) (PEO), to reduce crystallinity of PEO-based electrolytes [32]. Zhang et al. [33] reported the exceptional plasticization properties of polysorbates on carnauba wax, widely used in dentistry. Koocheki et al. [34] observed a clear decrease in Tg of poly(-d, l-lactide-co-glycolide) membranes for controlled drug release containing several amounts of Tween 80® (polyethylene glycol sorbitan monooleate). Yokesahachart et al. [35] reported the exceptional plasticization properties of Tween® 60 (polyethylene glycol sorbitan monostearate, 1.55 wt%) on plasticization of cassava starch, with improved processability. Sorbitan-based surfactants have been successfully used as plasticizers for cellulose esters with an exceptional decrease in Tg [31, 36]. Tween® 80 addition to PLA films obtained by solvent casting, led to lowering the glass transition temperature, Tg of neat PLA from 58.5 ºC down to values of 32 ºC for a polysorbate content of 20 wt%, as reported by Girdthep et al. [37].
This work proposes an alternative use of two different polysorbates, namely Tween® 20 (polyethylene glycol sorbitan monolaurate), and Tween® 80 (polyethylene glycol sorbitan monooleate), as environmentally friendly plasticizers for poly(lactide) (PLA) formulations for injection molding with improved ductility. The effect of the polysorbate type and amount on mechanical, thermal and thermomechanical properties is addressed, with the aim of widening the potential applications of these cost-effective materials in PLA plasticization.
Experimental
Materials
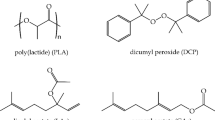
PLA used in this study was Purapol L130 supplied by Total Corbion PLA (Amsterdam, the Netherlands). It has a density of 1.24 g cm−3, an approximate molecular weight of 180 kg/mol and a 99% L-isomer content. Polysorbates, Tween® 20 and Tween® 80, were supplied by Sigma Aldrich (Madrid, Spain). Tween® 20 corresponds to a polyethylene glycol sorbitan monolaurate, with an average molecular weight of 1225 g mol−1, and a density of 1.095 g/cm3. On the other hand, Tween® 80 is the tradename of polyethylene glycol sorbitan monooleate, with a molecular weight of 1310 g mol−1, and a density of 1.064 g/cm3. Both contain an average number of ethylene oxide of 20 units per unit of sorbitol, while the main fatty acid is lauric and oleic acid for Tween® 20 and Tween® 80, respectively (see Fig. 1).
Processing of Plasticized PLA Formulations with Polysorbates
PLA pellets were first dried at 80 ºC for 12 h to remove residual moisture, since polyesters are very sensitive to hydrolysis at elevated processing temperatures. After this, pure PLA and PLA formulations with several compositions of each one of the polysorbates (10, 20 and 30 wt% of each) were weighed and pre-mixed in aluminium pods and fed into a 15-cc twin screw micro compounder Xplore MC 15 HT from Xplore Instruments BV (Sittard, The Netherlands). All formulations were mixed at a temperature of 190 ºC for 2 min, using a screw speed of 100 rpm. After this, the hot blends were charged into a removable barrel of a 12-cc micro injection molding machine Xplore IM 12 from Xplore Instruments BV (Sittard, The Netherlands), and injected into a mold cavity by the action of a plunger operated by compressed air, to obtain standard samples. The injection molding process was carried out at 190 ºC. After demolding, the samples were stored in a vacuum desiccator at room temperature for further characterization.
Mechanical Properties
Tensile properties of plasticized PLA formulations containing different amounts of polysorbates (Tween® 20 or Tween® 80) were obtained in a universal testing machine from S.A.E. Ibertest (Madrid, Spain), model IBERTEST DUOTRAC 10/1200 with a mounted load cell of 10 kN according to ISO 527-2:2012. The crosshead speed was set to 15 mm min−1 and the initial length was set to 50 mm to appropriately calculated the elongation at break after elastic recovery. In particular, the elastic modulus E, the maximum tensile strength (σmax), and the percentage of elongation at break (%εb) were determined for at least five different samples, and the average values of each tensile property, together with the standard deviation were obtained. Moreover, the work of fracture (WOF) was obtained by integrating the area under the stress-strain curve and averaged for at least five different samples.
The Shore D hardness of neat PLA and the plasticized formulations with polysorbates was obtained in a durometer from J. Bot S.A. (Barcelona, Spain), model 673-D, according to the guidelines of ISO 868:2003. At least, ten different measurements were made, and average Shore D values and standard deviations were obtained for each formulation.
Morphology
Morphology of the fractured impact test samples of neat PLA and plasticized PLA formulations with the polysorbates was assessed by field emission scanning electron microscopy (FESEM). Initially, the samples underwent sputter coating with a gold-palladium alloy using an EMITECH sputter coating mod. SC7620 from Quorum Technologies, Ltd. (East Sussex, UK). Subsequently, FESEM images were captured using a ZEISS ULTRA 55 microscope from Oxford Instruments (Abingdon, United Kingdom), operating at 2 kV.
Thermal Properties
The thermal properties of PLA and plasticized PLA formulations with polysorbates, were obtained by differential scanning calorimetry (DSC) in a DSC-821 calorimeter from Mettler-Toledo Inc. (Schwerzenbach, Switzerland) in nitrogen atmosphere (50 mL min−1). Samples with a weight in the 5–10 mg range were subjected to a first heating step from 25 to 180 ºC to remove previous thermal history at 10 ºC min−1. Then, a cooling step down to 0 ºC at -10 ºC min−1 was programmed to cool down the material in controlled conditions; finally, a second heating step was programmed up to 300 ºC at a heating rate of 10 ºC min−1. The main thermal parameters were obtained from this second heating cycle. In particular, the glass transition temperature (Tg), the cold crystallization peak temperature (Tcc) and the melting peak temperature (Tm) were obtained, as well as the enthalpies associated with the cold crystallization (ΔHcc) and the melting process (ΔHm). The degree of crystallinity developed during cooling (χc), and the maximum degree of crystallinity (χc_max) were obtained by using Eq. 1 and Eq. 2, respectively.
where ∆Hm and ∆Hcc stand for the melting and cold crystallization enthalpies of PLA, respectively, ∆Hm0 is the theoretical melting enthalpy of a fully crystalline PLA, that is, 93.7 J g−1 [38], and the term (1-w) represents the weight fraction of PLA in plasticized formulations.
It should be noted that the material has a specific degree of crystallinity when it is produced, and after the first heating cycle in DSC, the material is melted completely, erasing any thermal history. Then, the sample is cooled in a cooling cycle, where it can undergo melt crystallization. Finally, in the second heating cycle, it can also undergo cold crystallization process, and the Xc_max is the total crystalline fraction that includes both the melt crystallization (generated in the cooling cycle) and the cold crystallization (generated in the second heating cycle).
The thermal degradation of PLA and the plasticized formulations with polysorbates was followed by thermogravimetric analysis (TGA) in a TG-DSC2 thermobalance from Mettler Toledo (Columbus, Ohio, USA). A sample weight in the 7–10 mg range was placed into standard alumina cylindrical crucibles of 70 µL and covered with a lid. Then, the samples were subjected to a dynamic heating program from 30 ºC to 700 ºC under nitrogen atmosphere (50 mL min−1) at a heating rate of 20 ºC min−1. The main thermal parameters obtained by TGA were the onset degradation temperature (T5%) (taken as the temperature at which the material suffers a mass loss of 5%), and the maximum degradation rate temperature (Tmax), which corresponds to the peak temperature of the first derivative TGA curve or DTG. All samples characterized by DSC and TGA were analyzed in triplicate to obtain reliable results.
Thermo-Mechanical Characterization
Dynamic mechanical thermal analysis (DMTA) was carried out in a Mettler Toledo DMA1 (Columbus, Ohio, USA) under single cantilever conditions. The selected frequency was 1 Hz, and the maximum amplitude in the free cantilever was set to 10 µm. Samples with average dimensions of 25 × 10 × 4 mm3 were subjected to a dynamic heating ramp from − 50 ºC up to 100 ºC using a heating rate of 2 ºC min−1. The evolution of the storage modulus (E’), the loss modulus (E”), and the dynamic damping factor or tan δ, were recorded as a function of temperature. With regard to the dimensional stability of neat PLA and plasticized formulations containing polysorbates, thermomechanical analysis (TMA), was used to collect data of dimensional change with increasing temperature. A thermomechanical analyzer (TMA) model Q400 from TA Instruments (New Castle, Delaware, USA) was used. Samples with dimensions 10 × 10 × 4 mm3 were heated from − 50 ºC up to 120 ºC at a constant heating rate of 2 ºC min−1, with a constant force of 20 mN. The coefficient of linear thermal expansion or CLTE, was calculated as the slope in the linear regions below Tg and above cold crystallization to give a CLTE of the material in the glassy state (below Tg), and another CLTE in the rubbery-like state (above the cold crystallization). All TMA tests were run in triplicate to obtain reliable results.
Results and Discussion
Mechanical Properties of Plasticized PLA with Polysorbates
Figure 2 shows a comparative plot of the tensile stress-strain graph for neat PLA and plasticized PLA with different amounts of Tween® 20, and Tween® 80. As expected, neat PLA is a rather brittle polymer with a high tensile strength of about 78.7 MPa, a high tensile modulus of 2.1 GPa and a remarkable low elongation at break of 4.0%. Similar values have been reported in the literature having a tensile strength in the 40–70 MPa, and an elongation at break lower to 10%, which is responsible for low toughness and a brittle behavior. The addition of both Tween® 20 and Tween® 80, at lower concentrations of 10 wt% does not improve the ductile properties, but a decrease in tensile strength to values around 60 MPa is suggesting that some plasticization phenomenon is occurring, however, the amount of plasticizer is not high enough to trigger the plastic deformation mechanisms. Similar findings have been reported by Baiardo et al. [39] in plasticized PLA with different molecular weight polyethylene glycol (PEG) and acetyl tributyl citrate (ATBC) plasticizers. Despite the plasticization effects were confirmed by a decrease in the glass transition temperature, they did not observe a clear increase in elongation at break until a threshold plasticizer content was reached. In the case of ATBC, this threshold was 10 wt%. Below 10 wt% ATBC, the plasticized PLA showed an elongation at break of around 3%, while at slightly higher ATBC concentration of 12.5 wt%, the elongation at break dramatically increased up to 218%. They concluded that the exceptional elongation at break observed in different plasticized PLA formulations was related to the glass transition temperature (Tg). It was necessary to reach such a low Tg of 35 ºC to observe this exceptional increase in elongation at break. The results obtained in this work agree with this hypothesis and, as it will be discussed in the next section, plasticized PLA formulations containing 20 and 30 wt% polysorbates offer a Tg close to 35 ºC, or even lower. At a concentration of 20 wt%, polysorbates lead to a noticeable increase in elongation at break of around 180%, which is similar to the values reported by Greco et al. [40] in plasticized PLA containing 20 wt% cardanol, epoxidized cardanol acetate (ECA), and polyethylene glycol (PEG), thus confirming the exceptional plasticization effect polysorbates can provide to PLA in terms of ductility. As expected, the mechanical resistant properties, i.e. the maximum tensile strength (σmax), and the elastic modulus (E), decrease with increasing polysorbate content. It is important to remark that plasticized PLA formulations containing 30 wt% polysorbates, show σmax values of 23–24 MPa which are typical of some commodities. Recently, Barandiaran et al. [18] have reported this phenomenon on plasticized PLA with 20 wt% of different esters of cinnamic acid. As cinnamates are relatively low molecular weight plasticizers, they can enter into the amorphous regions and improve chain mobility in a greater extent than high molecular weight plasticizers such as polysorbates. Thus, the mechanical resistant properties are reduced in a more remarkable way, e.g. σmax changes from 52.7 MPa for neat PLA down to values of 14–20 MPa with different cinnamates. Moreover, if polysorbates are compared with traditional plasticizers for PLA, like epoxidized soybean oil (ESO), the elongation at break obtained in this study is quite superior to the elongation obtained by Xu et al. [41] for ESO plasticized PLA, where they reported a maximum elongation at break of 5.42% for an ESO content of 15 wt%. Additionally, polyethylene glycol (PEG), another traditional plasticizer for PLA, has been reported to provide PLA with an elongation at break of 96.6% at a composition of 20 wt%, which still is below the values reported for both polysorbates at 20 wt% [42], thereby showing the great potential this environmentally friendly polysorbates have as plasticizers for PLA.
Regarding Shore D hardness, the results shown in Table 1 follow the expected tendency. As the plasticizer content increases, Shore D hardness also decreases due to the improved ductile properties. By calculating the work of fracture as the area under the stress-strain curve, it is possible to observe in a clear way the remarkable improvement on toughness that polysorbates provide to PLA. PLA shows a very low work of fracture of 1.9 MJ m−3. Sing et al. [43] have reported a work of fracture of 1 MJ m−3 for PLA. Similar values have been reported by Harpool et al. [44] in 3D-printed PLA samples with different infill patterns. In this work, the addition of 10 wt% polysorbate does not improve the toughness and a slight decrease in the work of fracture is detected. Nevertheless, for a plasticizer concentration of 20 wt%, the work of fracture increases in a noticeable way up to values of 40–42 MJ m−3, while higher plasticizer concentration leads to a decrease in the work of fracture, thus suggesting that 20 wt% plasticizer content gives the best balance in mechanical properties. Zhang et al. [45] have reported a work of fracture or tensile toughness of 8.8 MJ m−3 for neat PLA. They studied the effect of natural rubber on tensile toughness by adding natural rubber in the 1–10 wt% range. They observed an increase in tensile toughness up to 35.6 MJ m−3 (with an elongation at break of 137.4%), for blends with 10 wt% mixed for 10 min. Ramlee et al. [46] also observed an increase in toughness from 6.7 MJ m−3 (neat PLA) up to 45.8 MJ m−3 in a binary blend of PLA and 50 wt% polyethylene carbonate (PEC). Therefore, the improvement on toughness that polysorbates provide to PLA are comparable to other different approaches made to overcome PLA brittleness.
Morphology of the Plasticized PLA Formulations with Polysorbates
In order to complement the mechanical properties analysis of the plasticized PLA samples, FESEM images were taken for all the samples at 500x magnification, which are gathered in Fig. 3. As it is observed, the morphology of neat PLA is quite flat with absence of indicatives of plastic deformation, as it is typical in this kind of polymer. This morphological behavior is indicative of a brittle fracture, and it is in total accordance with the low elongation at break shown in the previous Sect. (4%). Figure 3b and c show a very similar morphology, displaying a very flat surface with very little roughness, also indicating a very high brittleness, which corresponds to both samples with 10 wt% of each one of the polysorbates, which also presented a very brittle behavior with elongations at break very similar to that of neat PLA. On the other hand, Fig. 3d, e, f and g show a totally different morphology, with quite higher roughness compared to the previous samples, with a cavernous morphology and the appearance of filament-like structures that are indicative of a certain plastic deformation. This behavior demonstrates the plasticization effect exerted by both polysorbates at 20 and 30 wt% composition. These results are also in accordance with the mechanical results, which shown elongation at break values between 148 and 194% for these samples. Additionally, all the morphology images present an homogeneous structure, with little presence of particles, which confirms good compatibility and affinity between the polymer and the plasticizers. This good compatibility was also observed by Tan et al. [47] in PLA films plasticized with cellulose-based polypropoxy ether carboxylates (CPPEA), which also increased considerably the elongation at break of PLA, acting as effective plasticizers for this polymer.
Thermal Properties of Plasticized PLA with Polysorbates
In order to assess the thermal transitions of neat PLA and the plasticized PLA formulations differential scanning calorimetry (DSC) was carried out. Figure 4a shows the general thermograms obtained from this analysis from 0 to 220 ºC. Nonetheless, additional Figures have been constructed in order to focus more on each transition that occurs in the materials.
The effect of both polysorbates on the glass transition temperature of PLA is noticeable (Fig. 4b and c). The Tg of neat PLA, located at 61.0 ºC, is moved down to values of 29.5 ºC and 36.6 ºC for the plasticized formulations containing 30 wt% Tween® 20, and Tween® 80, respectively, thus giving clear evidence of the exceptional plasticization effectiveness of the proposed polysorbates.
This decrease is similar to that reported by Maiza et al. [48] in plasticized PLA formulations with acetyl tributyl citrate (ATBC), or triethyl citrate (TEC) at a concentration between 20 and 30 wt%. Fehri et al. [49] reported a decrease in the Tg of PLA from 59.4 ºC to 38.9 ºC with the addition of 15 wt% of a polyester derived from adipic acid and different alcohols. Litauszki et al. [50], reported the exceptional plasticization effects of oligomers of lactic acid (OLA) and adipates as effective plasticizers for PLA, with a noticeable decrease in Tg from 60 ºC (neat PLA with 1.4% D-lactide) to values comprised between 43 and 47 ºC with 10 wt% of the plasticizers mentioned above. This decrease in Tg is attributable to increased chain mobility due to the free volume provided by plasticizers [51]. As Tween® 20 has a slightly lower molecular weight compared to Tween® 80, its effect on lowering the Tg of PLA is slightly superior since low molecular weight plasticizers provide higher chain mobility than high molecular weight, oligomeric, or polymeric plasticizers as reported in literature with plasticizers that cover a wide range of molecular weights [18, 52,53,54,55]. If the reduction in Tg is compared to the results obtained for traditionally used plasticizers for PLA, such as ESO and PEG, Ozkoc et al. [42] reported a Tg of 29.4 ºC for PLA plasticized with 20 wt% of PEG, which is an incredible reduction, which in this case is very similar to the one reported for the sample plasticized with 30 wt% of Tween® 20. Ching et al. [56] reported a Tg of 45.8 ºC for a sample of PLA plasticized with 10 wt% of ESO, which is a value very similar to the ones herein reported for 10 wt% of Tween® 20 and Tween® 80 (47.1 and 47.3 ºC, respectively). This results demonstrate that both polysorbates are ideal candidates as environmentally friendly PLA plasticizers that can compete with traditional plasticizers.
The Fox Eq. (3) is one of the most used expressions to predict the glass transition temperature of plasticized polymers and polymer blends.
Where Tg stands for the glass transition of the plasticized PLA, Tg1 is the glass transition temperature of neat PLA, and w1 is its weight fraction, while Tg2 corresponds to the glass transition of Tween® 20 (-61 ºC), or Tween® 80 (-64 ºC), as reported in literature [25, 31], and w2, represents the plasticizer weight fraction in the mixture.
As it can be seen in Fig. 5a and b, despite the decreasing tendency of Tg, there is a positive deviation (the measured Tg is higher than the predicted Tg value by the Fox equation), which is higher as the weight fraction of the plasticizer increases. This phenomenon could be ascribed to stronger intermolecular interactions between the polymer and the plasticizer, which exceeds the simple additive rule of mixing expected by the Fox equation. As the plasticizer content increases, the intermolecular interactions become more significant, thus restricting the segmental mobility of the polymer chains, thereby increasing the Tg value with relation to the expected value. As a result, the polymer chains require more thermal energy than the expected energy by the additive rule to overcome these interactions and increase their mobility. Amim et al. [31], observed good agreement between the Fox equation and the experimental Tg values obtained for cellulose esters plasticized with different amounts of polysorbates. Despite good correlation was observed at plasticizer concentration below 30 wt% (Tween® 20, and Tween® 40), this threshold was lower with plasticized cellulose esters with Tween® 60 (below 10 wt%). As they concluded, as smaller is the plasticizer molecule, the larger is the weight% plasticizer with good agreement to Fox equation. The obtained results in plasticized PLA with polysorbates, agrees to this phenomenon since the plasticized PLA formulations with Tween® 20 (lower molecular weight-smaller molecule) offer less deviations than formulations with Tween® 80 (with higher molecular weight). Courgneau et al. [57], have reported the same phenomenon on plasticized PLA with acetyl tributyl citrate (ATBC), with experimental Tg values higher than those predicted by the Fox equation. They also observed that the deviation was higher as the plasticizer weight fraction increased. Pillin et al. [58], also described this behaviour in plasticized PLA with different molecular weight polyethylene glycol (PEG). They observed good agreement at low plasticizer content, while the lowest deviation between the theoretical values and the experimental values was obtained for plasticized formulations with the lowest molecular weight PEG, thus giving coherence to the obtained results in plasticized PLA with polysorbates.
With regard to the cold crystallization (see Fig. 6) phenomenon (cold crystallization peaks were obtained from the second heating cycle), both polysorbates shift the cold crystallization peak temperature (Tcc) to lower values (see Table 2) due to the increased mobility provided by the flexible plasticizer molecules, as suggested by Maiza et al. [59] in plasticized PLA formulations with different citrate esters. This phenomenon has also been reported by Li et al. [55] in plasticized PLA with poly(ethylene glycol) (PEG) with different molecular weights (400–4000 g mol−1). They observed PEG with a molecular weight of 400 g mol−1, led to the highest decrease in both Tg and the cold crystallization peak temperature (Tcc). In this work, Tcc changes from 106.7 ºC (neat PLA) down to such low values of 72.6 ºC and 80.0 ºC for the plasticized formulations containing 30 wt% Tween® 20, and Tween® 80, respectively. Another important issue related to the cold crystallization is the peak shape as it becomes narrower with increasing the polysorbate amount. This narrow peak shape can be observed in Fig. 6, as well as in Table 2, since the full-width at half-maximum (FWHM) of the peak decreases as the plasticizer content increases for both polysorbates. As this shift of the cold crystallization is related to increased chain mobility, it is Tween® 20 (with lower molecular weight) which gives the highest decrease in Tcc. It has been proposed that plasticizers could enhance crystallization at lower temperatures due to improved chain mobility, and hence, PLA crystallization becomes easier [60].
With regard to the effect of polysorbates on the melting process, it is evident both polysorbates promote some interesting changes (see Fig. 7) in the shape and maximum peak. In particular, the melting peak temperature (Tm) is slightly reduced from 171.7 ºC for neat PLA down to 165.6 ºC (formulation with 30 wt% Tween® 20), or 167.7 ºC (formulation with 30 wt% Tween® 80). This phenomenon was more pronounced in plasticized PLA with different citrate esters as reported by Maiza et al. [59], with a decrease of Tm of neat PLA from approximately 165 ºC down to such low values of 138.55 ºC (30 wt% triethyl citrate, TEC). They ascribed this decrease in Tm to the ability of citrate esters to destroy de crystal structure of PLA. Plasticizer addition has also a pronounced effect on the degree of crystallinity (%χc) as can be seen in Table 3. The degree of crystallinity (%χc) developed by PLA after cooling is 1.9% and its maximum degree of crystallinity (%χc_max), is 33.9% which means, most of its crystallinity is developed during the cold crystallization process, as when the polymer undergoes the second heating cycle, only 1.9% of the maximum crystallinity corresponds to organized polymer chains before starting the second heating cycle. As the plasticizer content increases, the degree of crystallinity (%χc) dramatically increases up to 22–23% for a plasticizer content of 30 wt% (both polysorbates). This is related to the increased chain mobility provided by the plasticizer molecules as reported by Xiao et al. [61], in plasticized PLA formulations with 10–30 wt% triphenyl phosphate (TPP). They attributed this increase in crystallinity to improved segmental molecular mobility of PLA which makes easier crystallization in plasticized formulations. They also observed an increase in the degree of crystallinity developed during the cold crystallization process which lead to maximum degree of crystallinity values of 52.5% for the plasticized formulation containing 30 wt% TPP which is very similar to the values obtained in this study with %χc_max values of 49–50% for both polysorbates with the same concentration (i.e. 30 wt%). This phenomenon was also observed by Ljungberg et al. [62], in plasticized PLA formulations with several plasticizers with a fixed concentration of 15 wt%, including tributyl citrate (TbC), diethyl bishydroxymethyl malonate (DBM), and their oligomers. The decrease in the melt peak temperature provided by the plasticizers was similar to the change observed in this study, and all plasticizers lead to increased degree of crystallinity.
With regard to the thermal stability, one critical concern on the use of monomeric plasticizers is the potential plasticizer loss during processing, due to the low molecular weight of most monomeric plasticizers. This can be assessed by thermogravimetric analysis (TGA). PLA-based formulations are usually processed at temperatures ranging from 190 to 210 ºC, which could lead to some plasticizer loss as reported by Arrieta et al. [16], in plasticized PLA formulations with limonene (boiling point at 176 ºC). Ivorra-Martinez et al. [30] have also reported this phenomenon on plasticized PLA with dibutyl itaconate (DBI) (boiling point at 284 ºC). Recently, Gomez-Caturla et al. [17] have shown the potential of geraniol esters as environmentally friendly plasticizers for PLA. In spite of the fact that the boiling point of these geraniol esters is comprised between 216 ºC (geranyl formate with a molecular weight of 182.3 g mol−1) and 276–277 ºC (geranyl isovalerate with a higher molecular weight of 238.4 g mol−1), they observed improved thermal stability (by measuring the onset degradation temperature) by increasing the molecular weight. In this work, both polysorbates show a chemical structure that could be classified in the threshold between low molecular weight monomeric plasticizers and high molecular weight oligomeric/polymeric plasticizers. Tween® 20 has a molecular weight of 1225 g mol−1, while Tween® 80 has higher molecular weight of 1310 g mol−1. The effect of these polysorbates on the thermal stability of the plasticized PLA formulations with varying plasticizer content, can be observed in Fig. 8a-c, while the first derivative curve profiles (DTG) is gathered in Fig. 8b-d. The most relevant parameters regarding the thermal degradation are summarized in Table 4.
As it can be seen in Fig. 8a-c, the thermal degradation of neat PLA occurs in a single step process with an onset degradation temperature, T5% (temperature for a mass loss of 5 wt%) of 304 ºC, and a maximum degradation rate temperature (Tmax) of 343 ºC. The thermal degradation of plasticized PLA formulations with Tween® 20 and Tween® 80 also occurs in a single step process. Many authors have reported a two-step degradation process in plasticized PLA formulations, the first step consisting on the plasticizer loss at lower temperatures than the high molecular weight PLA chains. Maiza et al. [48] observed this phenomenon on plasticized PLA with triethyl citrate (TEC) and acetyl tributyl citrate (ATBC). They reported a decrease in the onset degradation temperature (T5%) from 295 ºC (neat PLA) down to such low values of 141 ºC and 146 ºC for plasticized PLA formulations containing 30 wt% TEC and ATBC, respectively. In this particular case, the thermal degradation occurs in a single step probably due to the fact that Tween® 20 and Tween® 80 degrade in the same temperature range than PLA, as it was reported by Bide et al. [63], who showed that Tween® 80 thermodegrades in a single step between 350 and 400 ºC, which is the same temperature span than for PLA. An additional study performed by Kishore et al. [64] also showed that Tween® 20 and Tween® 80 thermodegrade in a temperature range between 350 and 400 ºC.
Moreover, the TGA degradation profile changed from a single step process for neat PLA to a several step degradation for both TEC and ATBC plasticizers, due to the higher volatility of these plasticizers compared to PLA. Similar findings were reported by Barandiaran et al. [18] in plasticized PLA with esters of cinnamic acid. In fact, as the TGA curves of plasticized PLA with cinnamates showed a two-step degradation, the plasticizer loss during processing was assessed by measuring mass loss related to the first degradation step observed by TGA. Arrieta et al. [65] also observed this two-step degradation process in PLA-limonene and PHB-limonene blends. On the other hand, Burgos et al. [66] reported a single step degradation process for PLA and plasticized PLA with oligomers of lactic acid (OLA with a molecular weight of 957 g mol−1), with a decrease in the onset degradation temperature (T5%) with increasing the plasticizer content, thus showing that thermal degradation of high molecular weight plasticizers overlaps with that of PLA. Similar findings have been reported by Lascano et al. [52] in plasticized PLA with OLA for improved toughness. In this work, polysorbates show a molecular weight above 1200 g mol−1, and accordingly to other oligomeric plasticizers, the degradation process proceeds in a single step process with a decrease in the onset degradation temperature (T5%) with increasing plasticizer content, as summarized in Table 4. Therefore, for the plasticized PLA formulation containing Tween® 20, the T5% changes from 304 ºC (neat PLA) down to 271 ºC (plasticized PLA with 30 wt% Tween® 20). Similar tendency can be observed for plasticized PLA formulations containing Tween® 80, with slightly higher T5% values since it has higher molecular weight than Tween® 20.
Thermo-mechanical Properties of Plasticized PLA with Polysorbates
Figure 9 gathers dynamic-mechanical thermal analysis (DMTA) graphs (storage modulus vs temperature, and tan δ vs temperature) for PLA and plasticized PLA formulations with polysorbates. The evolution of the storage modulus (E’) with temperature can be seen in Fig. 9a and c, for plasticized PLA formulations containing Tween® 20, and Tween® 80, respectively. Several processes can be observed for neat PLA as reported by Cristea et al. [67]. At low temperatures (below 50 ºC), the change in E’ with temperature is almost negligible. In this temperature range, PLA shows a brittle behavior since this temperature range corresponds to its glassy region. In the 50–75 ºC temperature range, a three-fold decrease in E’ can be observed, which is attributable to the glass transition temperature (Tg). At 75–80 ºC, a rubbery plateau region can be seen but, immediately above 80 ºC, the cold crystallization process can be observed by an increase in E’, since cold crystallization leads to a more packed structure which, in turn, increases the resistance and modulus. After 80 ºC, a second rubbery plateau region can be seen. These regions agree with those reported by Ferri et al. [68] for neat PLA and binary blends with thermoplastic starch (TPS). The evolution of the storage modulus, E’, with temperature for plasticized PLA formulations with polysorbates shows identical shape, but typical curves are shifted to lower temperatures. Consequently, the above-mentioned processes (glass transition, and cold crystallization) are moved to lower temperatures. Moreover, as the plasticizer content increases, the shift to lower temperatures is more pronounced. These results are in total agreement with those observed by DSC. As can be seen in Fig. 9a and c, the first rubbery plateau region, comprised between the glass transition and the cold crystallization, is very narrow for neat PLA but in plasticized PLA formulations with polysorbates too. This has been explained by Cristea et al. [67], as a sudden increase in E’ just after the glass transition that may be due to the fact that the glass transition can trigger the cold crystallization due to the increased chain motion. The onset of the cold crystallization (Tcc_onset) has been determined as this sudden increase in E’ after the glass transition. As expected, the onset of the cold crystallization is moved down to lower values as the plasticizer content increases, with similar trend with both polysorbates and in agreement with the second heating in the previous characterization by DSC. The characterization in DMTA is related to the first heating of the material, nonetheless, it helps to better understand the cold crystallization phenomenon in general terms.
Despite there are several criteria to identify the glass transition temperature (Tg) by means of DMTA, the peak maximum of the dynamic damping factor (tan δ peak), is usually employed (see Table 5). As can be seen in Fig. 9b, d and Tg decreases with increasing plasticizer content for both polysorbates. More specifically, the Tg of neat PLA, located at 63.9 ºC, decreases to values of around 49 ºC in plasticized formulations with 30 wt% Tween® 20, and Tween® 80, thus showing the high plasticization efficiency polysorbates can exert on PLA. Notwithstanding the Tg values are different to those obtained by differential scanning calorimetry (DSC) due to the different thermal history, as it has been previously commented, the same decreasing tendency can be observed. As the plasticizer content increases for both polysorbates, additional changes in the dynamic damping factor peak occur. In particular, the peak height decreases and the peak shape is broader. The peak shape is assessed by the full-with at half-maximum (FWHM), as shown in Table 5. PLA shows a narrow tan d peak, with a FWHM value of 8.5 ºC, while the plasticized PLA formulations containing 30 wt% Tween® 20, and Tween® 80, show a broader peak characterized by a FWHM of 12.6 ºC for both polysorbates. Similar tendency on the dynamic damping factor has been reported by Ren et al. [69] in plasticized PLA with triacetine (TAC) and poly(1,3-butylene glycol adipate) (PBGA). Ivorra-Martinez et al. [30] have reported this phenomenon in plasticized PLA with dibutyl itaconate (DBI). They concluded that this broadening highly depends on the molecular weight and the plasticizer amount. Since DBI has a low molecular weight (242.32 g mol−1), the FWHM reaches such high values of 85.2 ºC at a nominal concentration of 20 wt% DBI. This phenomenon is related to the micro heterogeneities generated by the presence of plasticizer molecules inside the amorphous regions of PLA. As a consequence, the interactions between PLA chains and PLA-plasticizer are affected, leading to a broader temperature range for the glass transition temperature, Tg [18]. As concluded by Galvez et al. [70], a higher FWHM value is representative for more interaction and contact between PLA and plasticizer molecules, which leads to a wide range of relaxation times. As low molecular weight molecules can enter easily into the amorphous regions, this phenomenon is more pronounced with low molecular weight plasticizers. As polysorbates have a molecular weight in the threshold of monomeric plasticizers (usually below 600 g mol−1) and oligomeric/polymeric plasticizers (ranging from 1000 to 10000 g mol−1, with an average of 3000 g mol−1) [71], they promote this broadening phenomenon in a less extent than low molecular weight monomeric plasticizers such as triethyl citrate (TEC) and acetyl tributyl citrate (ATBC) with FWHM values of 50–60 ºC at a plasticizer content of 30 wt% [59]. Additionally, some peaks in the tan d graphs can be observed between 60 and 100 ºC. These peaks are related to the cold crystallization transition, which is observed as an increase in the storage modulus as a result of an increase in the rigidity of the materials due to crystallization. This phenomenon was observed also by Ivorra-Martinez et al. [30] in DBI-PLA plasticized samples. It is also seen how the higher the polysorbate content, the lower the temperature at which these peaks occur, which is in total accordance with the DSC analysis previously commented.
With regard to the dimensional stability, thermomechanical analysis (TMA) gives interesting results about the effect of polysorbate-type plasticizers on PLA. Figure 10 depicts the dimensional change in plasticized PLA formulations containing different weight fractions of Tween® 20 (Fig. 10a), and Tween® 80 (Fig. 10b). The main results obtained by TMA are gathered in Table 6. The dimensional change of PLA with temperature is very helpful to understand all the occurring phenomena in these materials. Below its Tg (temperature range below 50 ºC), there is a linear correlation between the increase in temperature and the increase in dimensional change. The glass transition phenomenon is related to a softening, thus leading to increased deformation (expansion) as a consequence of the temperature rise. Thus, the glass transition temperature can be detected as the change in the slope of the linear region at temperatures below Tg (see orange arrows in Fig. 10).
On the other hand, at temperatures above Tg, PLA undergoes cold crystallization, which leads to a more packed structure. Subsequently, this phenomenon is observed by TMA as a decrease in the dimensional change (contraction) due to the fact that packed PLA chains occupy less volume. Due to the glass transition phenomenon and the cold crystallization processes are overlapped, it is possible to identify the onset of the glass transition temperature (orange arrows), and the endset of the cold crystallization (black arrows). As reported by Cristea et al. [67], the glass transition can trigger the cold crystallization and the effects of these two phenomena on the dimensional change are overlapped, as seen in Fig. 10. As the plasticizer content increases for both Tween® 20 and Tween® 80, the glass transition temperature onset is moved down to lower values, according to the results obtained by DSC and DMTA. Consistently, the end of the cold crystallization also moves to lower temperatures as previously observed. The effect of the plasticizer type and weight fraction on the coefficient of linear thermal expansion (CLTE) has been assessed by thermomechanical analysis. This overlapping has been described by Ivorra-Martinez et al. [30] in plasticized PLA with different amounts of dibutyl itaconate (DBI). Since the glass transition and the cold crystallization are overlapped, the CLTE has been measured as the slope of the linear region before the glass transition phenomenon (glassy state), and above the end of the cold crystallization (rubbery state). Neat PLA has a CLTE below Tg of 76 μm m−1 ºC−1, and this increases with increasing the plasticizer weight fraction since plasticizer molecules enter into the amorphous regions, thus weakening the polymer-polymer interactions which in turn, leads to an increase in the dimensional change with temperature as reported by Balart et al. [72] in plasticized PLA with epoxidized linseed oil (ELO). The effect both polysorbates is similar as can be seen in Table 6. Above the cold crystallization, the CLTE is higher due to the material is in a rubbery-like state and, subsequently, the dimensional change with temperature is higher. In the case of neat PLA, the CLTE above the cold crystallization is 161 μm m−1 ºC−1, and in a similar way, as the plasticizer content increases, the CLTE increases too. Similar findings have been reported by Quiles-Carrillo et al. [73], in plasticized PLA with different weight fractions of maleinized hempseed oil (MHO).
Conclusions
Polysorbates have demonstrated exceptional plasticization properties on poly(lactide) (PLA) matrix, thus widening their industrial applications in addition to their surfactant properties. Polysorbates, with a molecular weight comprised between 1200 and 1300 g mol−1, are in the frontier between monomeric and polymeric plasticizers, thus offering the benefits of both types of plasticizers. Moreover, due to their surfactant properties, they show good miscibility with PLA, much higher to that of similar molecular weight epoxidized vegetables oils. This miscibility leads to a remarkable increase in ductile properties. The elongation at break of the brittle, low-toughness PLA, is around 4.0% and is increased up to values of 180% in formulations containing 20 wt% of polysorbates. The plasticization efficiency of polysorbates has been assessed by measuring the glass transition temperature, Tg. PLA-polysorbate blends give a unique Tg which decreases with increased plasticizer content. Moreover, the cold crystallization is moved down to lower temperatures since the plasticizer molecules improve chain mobility. The maximum degree of crystallinity PLA can develop after cooling plus a cold crystallization also increases in a noticeable way, thus allowing potential annealing processes to tailor the desired mechanical and thermal properties. As polysorbates are medium-molecular weight organic molecules, they are not highly volatile which provides plasticized PLA formulations with good thermal stability. It has been noted that the low molecular weight polysorbate (Tween® 20) has slightly higher plasticization efficiency than the high molecular weight polysorbate (Tween® 80). This research widens the potential of a new series of plasticizers based on polysorbates, with interesting properties including cost-effective, readily available, and environmental issues since they can be obtained from renewable resources.
Data Availability
No datasets were generated or analysed during the current study.
References
Chai H, Chang Y, Zhang Y, Chen Z, Zhong Y, Zhang L, Sui X, Xu H, Mao Z (2020) The fabrication of polylactide/cellulose nanocomposites with enhanced crystallization and mechanical properties. Int J Biol Macromol 1551578–1551588
Kakroodi AR, Kazemi Y, Ding W, Ameli A, Park CB (2015) Poly (lactic acid)-based in situ microfibrillar composites with enhanced crystallization kinetics, mechanical properties, rheological behavior, and foaming ability. Biomacromolecules 16(12):3925–3935
Gomez-Caturla J, Tejada-Oliveros R, Ivorra-Martinez J, Garcia-Sanoguera D, Balart R, Garcia-Garcia D (2024) Development and characterization of new environmentally friendly polylactide formulations with terpenoid-based plasticizers with improved ductility. J Polym Environ 32(2):749–762
Chen C, Tian Y, Li F, Hu H, Wang K, Kong Z, Ying WB, Zhang R, Zhu J (2020) Toughening polylactic acid by a biobased poly (butylene 2, 5-furandicarboxylate)-b-poly (ethylene glycol) copolymer: balanced mechanical properties and potential biodegradability. Biomacromolecules 22(2):374–385
Zhang MY, Chang ZH, Wang XF, Li Q (2021) Synthesis of poly(l-lactide-co-ε-caprolactone) copolymer: structure, toughness, and elasticity. Polymers 13(8)
Jing ZX, Shi XT, Zhang GC (2017) Synthesis and properties of biodegradable supramolecular polymers based on polylactide-block-poly(δ-valerolactone)-block-polylactide triblock copolymers. Polym Int 66(11):1487–1497
Jeong J, Yoon S, Yang X, Kim YJ (2023) Super-tough and biodegradable poly(lactide-co-glycolide) (PLGA) transparent thin films toughened by star-shaped PCL- b-PDLA plasticizers. Polymers 15(12)
Li JJ, Guo SZ, Wang M, Ye L, Yao FL (2015) Poly(lactic acid)/poly(ethylene glycol) block copolymer based shell or core cross-linked micelles for controlled release of hydrophobic drug. RSC Adv 5(25):19484–19492
Puthumana M, Krishnan PSG, Nayak SK (2020) Chemical modifications of PLA through copolymerization. Int J Polym Anal Charact 25(8)
Llanes LC, Clasen SH, Pires AT, Gross IP (2021) Mechanical and thermal properties of poly (lactic acid) plasticized with dibutyl maleate and fumarate isomers: promising alternatives as biodegradable plasticizers. Eur Polymer J 142110112
Gross IP, Saatkamp RH, Sanches MP, Parize AL, Pires AT (2022) Poly (lactic acid)/poly (vinyl alcohol) biodegradable blends using monobutyl maleate as a plasticizer and compatibilizer. ACS Appl Polym Mater 5(1):99–108
Frone AN, Popa MS, Usurelu CD, Panaitescu DM, Gabor AR, Nicolae CA, Raduly MF, Zaharia A, Alexandrescu E (2022) Bio-based poly(lactic acid)/Poly(butylene sebacate) blends with improved toughness. Polymers 14(19)
Kong J, Li Y, Bai Y, Li Z, Cao Z, Yu Y, Han C, Dong L (2018) High-performance biodegradable polylactide composites fabricated using a novel plasticizer and functionalized eggshell powder. Int J Biol Macromol 11246–11253
Safari M, Kasmi N, Pisani C, Berthé V, Müller AJ, Habibi Y (2022) Effect of the structural features of biobased linear polyester plasticizers on the crystallization of polylactides. Int J Biol Macromol 214128–214139
Deokar MD, Garnaik B, Sivaram S (2022) Toughening poly(L-lactide) blends: effectiveness of sequence-controlled six-arm star-branched Block copolymers of Poly(L-lactide) and poly(ε-caprolactone). Acs Omega 7(11):9118–9129
Arrieta MP, López J, Ferrándiz S, Peltzer MA (2013) Characterization of PLA-limonene blends for food packaging applications. Polym Test 32(4):760–768
Gomez-Caturla J, Ivorra-Martinez J, Tejada-Oliveros R, Moreno V, Garcia-Garcia D, Balart R (2024) Effect of the chain length of geraniol esters on the plasticization efficiency with poly(lactide). Polymer 290126522
Barandiaran A, Gomez-Caturla J, Ivorra-Martinez J, Lascano D, Selles MA, Moreno V, Fenollar O (2023) Esters of Cinnamic Acid as Green Plasticizers for Polylactide Formulations with Improved Ductility, Macromolecular Materials and Engineering
Singh AA, Sharma S, Srivastava M, Majumdar A (2020) Modulating the properties of polylactic acid for packaging applications using biobased plasticizers and naturally obtained fillers. Int J Biol Macromol 1531165–1531175
Arrieta MP, Samper MD, López J, Jiménez A (2014) Combined effect of poly (hydroxybutyrate) and plasticizers on polylactic acid properties for film intended for food packaging. J Polym Environ 22460–22470
Bouti M, Irinislimane R, Belhaneche-Bensemra N (2022) Properties investigation of epoxidized sunflower oil as bioplasticizer for poly (lactic acid). J Polym Environ 30(1):232–245
Tan J, Huang N, Wu J, Xu M, Wang W, Wang X, Zhu X (2023) Synthesis of gallic acid-derived plasticizers for polyvinyl chloride featuring excellent plasticization, thermo-stability, and migration resistance. ACS Appl Polym Mater 5(10):8608–8617
Tan J, Zhang T, Wang F, Huang N, Yu M, Wei L, Jia P, Zhu X (2022) One-pot and industrial manufacturing of cardanol-based polyoxyethylene ether carboxylates as efficient and improved migration resistance plasticizers for PVC. J Clean Prod 375133943
Xie D, Zhao Y, Li Y, LaChance AM, Lai J, Sun L, Chen J (2019) Rheological, thermal, and degradation properties of PLA/PPG blends. Materials 12(21):3519
Lind TK, Nilsson EJ, Wyler B, Scherer D, Skansberger T, Morin M, Kocherbitov V, Engblom J (2021) Effects of ethylene oxide chain length on crystallization of polysorbate 80 and its related compounds. J Colloid Interface Sci 592468–592484
Merle J, Trinsoutrot P, Charrier-El F, Bouhtoury (2016) Optimization of the formulation for the synthesis of bio-based foams. Eur Polymer J 84577–84588
Thauvin C, Schwarz B, Delie F, Allémann E (2018) Functionalized PLA polymers to control loading and/or release properties of drug-loaded nanoparticles. Int J Pharm 548(2):771–777
Shakeel F, Alshehri S, Ibrahim MA, Altamimi M, Haq N, Elzayat EM, Shazly GA (2021) Solubilization and thermodynamic properties of simvastatin in various micellar solutions of different non-ionic surfactants: computational modeling and solubilization capacity. PLoS ONE 16(4)
Agrawal A, Saran AD, Rath SS, Khanna A (2004) Constrained nonlinear optimization for solubility parameters of poly(lactic acid) and poly(glycolic acid) - validation and comparison. Polymer 45(25):8603–8612
Ivorra-Martinez J, Peydro MA, Gomez-Caturla J, Boronat T, Balart R (2022) The potential of an Itaconic Acid Diester as environmentally friendly plasticizer for injection-molded Polylactide Parts. Macromol Mater Eng 307(12)
Amim J, Blachechen LS, Petri DFS (2012) Effect of sorbitan-based surfactants on glass transition temperature of cellulose esters. J Therm Anal Calorim 107(3):1259–1265
Mishra K, Pundir SS, Rai DK (2017) Effect of polysorbate plasticizer on the structural and ion conduction properties of PEO-NH4PF6 solid polymer electrolyte. Ionics 23(1):105–112
Zhang Y, Adams MJ, Zhang ZB, Vidoni O, Leuenberger BH, Achkar J (2016) Plasticisation of carnauba wax with generally recognised as safe (GRAS) additives. Polymer 86208–86219
Koocheki S, Madaeni SS, Niroomandi P (2011) Development of an enhanced formulation for delivering sustained release of buprenorphine hydrochloride. Saudi Pharm J 19(4):255–262
Yokesahachart C, Yoksan R (2011) Effect of amphiphilic molecules on characteristics and tensile properties of thermoplastic starch and its blends with poly(lactic acid). Carbohydr Polym 83(1):22–31
Amim J, Kawano Y, Petri DFS (2009) Thin films of carbohydrate based surfactants and carboxymethylcellulose acetate butyrate mixtures: morphology and thermal behavior. Mater Sci Eng C-Biomimetic Supramolecular Syst 29(2):420–425
Girdthep S, Punyodom W, Molloy R, Channuan W (2011) Effect of Tween 80 on the mechanical and thermal properties of solution-cast blends of poly(lactic acid) and cellulose acetate butyrate films. In: International Conference on Chemistry and Chemical Process (ICCCP 2011), Bangkok, THAILAND, pp. 95–100
Nagarajan V, Mohanty AK, Misra M (2016) Crystallization behavior and morphology of polylactic acid (PLA) with aromatic sulfonate derivative. J Appl Polym Sci 133(28)
Baiardo M, Frisoni G, Scandola M, Rimelen M, Lips D, Ruffieux K, Wintermantel E (2003) Thermal and mechanical properties of plasticized poly(L-lactic acid). J Appl Polym Sci 90(7):1731–1738
Greco A, Ferrari F (2021) Thermal behavior of PLA plasticized by commercial and cardanol-derived plasticizers and the effect on the mechanical properties. J Therm Anal Calorim 146(1):131–141
Xu YQ, Qu JP (2009) Mechanical and rheological properties of epoxidized soybean oil plasticized poly (lactic acid). J Appl Polym Sci 112(6):3185–3191
Ozkoc G, Kemaloglu S (2009) Morphology, biodegradability, mechanical, and thermal properties of nanocomposite films based on PLA and plasticized PLA. J Appl Polym Sci 114(4):2481–2487
Singh S, Patel MK, Geng SY, Teleman A, Herrera N, Schwendemann D, Maspoch ML, Oksman K (2021) Orientation of polylactic acid-chitin nanocomposite films via combined calendering and uniaxial drawing: effect on structure, mechanical, and thermal properties. Nanomaterials 11(12)
Harpool TD, Alarifi IM, Alshammari BA, Aabid A, Baig M, Malik RA, Sayed AM, Asmatulu R, El-Bagory T (2021) Evaluation of the infill design on the tensile response of 3d printed polylactic acid polymer. Materials 14(9)
Zhang CM, Huang Y, Luo CH, Jiang L, Dan Y (2013) Enhanced ductility of polylactide materials: reactive blending with pre-hot sheared natural rubber. J Polym Res 20(4)
Ramlee NA, Tominaga Y (2019) Mechanical and degradation properties in alkaline solution of poly(ethylene carbonate)/poly(lactic acid) blends. Polymer 16644–16649
Tan J, Yu M, Zhang T, Huang N, Cao Z, Wei L, Zhu X (2023) Cellulose-based polypropoxy ether carboxylates as highly compatible, effective, and migration-resistant plasticizers for poly (lactic acid). Int J Biol Macromol 253127675
Maiza M, Benaniba MT, Quintard G, Massardier-Nageotte V (2015) Biobased additive plasticizing polylactic acid (PLA). Polimeros-Ciencia E Tecnologia 25(6):581–590
Fehri S, Cinelli P, Coltelli MB, Anguillesi I, Lazzeri A (2016) Thermal properties of Plasticized Poly (Lactic Acid) (PLA) containing Nucleating Agent. Int J Chem Eng Appl 7(2):85–88
Litauszki K, Petrrény R, Haramania Z, Mészáros L (2023) Combined effects of plasticizers and D-lactide content on the mechanical and morphological behavior of polylactic acid. Heliyon 9(4)
Tabi T, Ageyeva T, Kovacs JG (2022) The influence of nucleating agents, plasticizers, and molding conditions on the properties of injection molded PLA products. Mater Today Commun 32
Lascano D, Moraga G, Ivorra-Martinez J, Rojas-Lema S, Torres-Giner S, Balart R, Boronat T, Quiles-Carrillo L (2019) Development of injection-molded polylactide pieces with high toughness by the addition of lactic acid oligomer and characterization of their shape memory behavior. Polymers 11(12)
Gomez-Caturla J, Dominguez-Candela I, Medina-Casas MP, Ivorra-Martinez J, Moreno V, Balart R, Garcia-Garcia D (2023) Improvement of poly(lactide) ductile properties by plasticization with biobased tartaric acid ester. Macromol Mater Eng 308(7)
Gzyra-Jagiela K, Sulak K, Draczynski Z, Podzimek S, Galecki S, Jagodzinska S, Borkowski D (2021) Modification of poly(lactic acid) by the plasticization for application in the packaging industry. Polymers 13(21)
Li DC, Jiang Y, Lv SS, Liu XJ, Gu JY, Chen QF, Zhang YH (2018) Preparation of plasticized poly (lactic acid) and its influence on the properties of composite materials. PLoS ONE 13(3)
Xing C, Matuana LM (2016) Epoxidized soybean oil-plasticized poly (lactic acid) films performance as impacted by storage. J Appl Polym Sci 133(12)
Courgneau C, Domenek S, Guinault A, Avérous L, Ducruet V (2011) Analysis of the structure-properties relationships of different Multiphase systems based on Plasticized Poly(Lactic Acid). J Polym Environ 19(2):362–371
Pillin I, Montrelay N, Grohens Y (2006) Thermo-mechanical characterization of plasticized PLA: is the miscibility the only significant factor? Polymer 47(13):4676–4682
Maiza M, Benaniba MT, Massardier-Nageotte V (2016) Plasticizing effects of citrate esters on properties of poly(lactic acid). J Polym Eng 36(4):371–380
Vieira MGA, da Silva MA, dos Santos LO, Beppu MM (2011) Natural-based plasticizers and biopolymer films: a review. Eur Polymer J 47(3):254–263
Xiao HW, Lu W, Yeh JT (2009) Effect of plasticizer on the crystallization behavior of poly(lactic acid). J Appl Polym Sci 113(1):112–121
Ljungberg N, Wesslén B (2005) Preparation and properties of plasticized poly(lactic acid) films. Biomacromolecules 6(3):1789–1796
Bide Y, Fashapoyeh MA, Shokrollahzadeh S (2021) Structural investigation and application of tween 80-choline chloride self-assemblies as osmotic agent for water desalination. Sci Rep 11(1):17068
Kishore RS, Pappenberger A, Dauphin IB, Ross A, Buergi B, Staempfli A, Mahler H-C (2011) Degradation of polysorbates 20 and 80: studies on thermal autoxidation and hydrolysis. J Pharm Sci 100(2):721–731
Arrieta MP, López J, Hernández A, Rayón E (2014) Ternary PLA-PHB-Limonene blends intended for biodegradable food packaging applications. Eur Polymer J 50255–50270
Burgos N, Martino VP, Jiménez A (2013) Characterization and ageing study of poly(lactic acid) films plasticized with oligomeric lactic acid. Polym Degrad Stab 98(2):651–658
Cristea M, Ionita D, Iftime MM (2020) Dynamic mechanical analysis investigations of PLA-based renewable materials: how are they useful? Materials 13(22)
Ferri JM, Garcia-Garcia D, Carbonell-Verdu A, Fenollar O, Balart R (2018) Poly(lactic acid) formulations with improved toughness by physical blending with thermoplastic starch. J Appl Polym Sci 135(4)
Ren ZJ, Dong LS, Yang YM (2006) Dynamic mechanical and thermal properties of plasticized poly(lactic acid). J Appl Polym Sci 101(3):1583–1590
Gálvez J, Aguirre JPC, Salazar MAH, Mondragón BV, Wagner E, Caicedo C (2020) Effect of Extrusion Screw Speed and Plasticizer proportions on the Rheological, Thermal, mechanical, morphological and superficial properties of PLA. Polymers 12(9)
Godwin AD (2017) Plasticizers, applied plastics engineering handbook. pp 533–553
Balart JF, Fombuena V, Fenollar O, Boronat T, Sánchez-Nacher L (2016) Processing and characterization of high environmental efficiency composites based on PLA and hazelnut shell flour (HSF) with biobased plasticizers derived from epoxidized linseed oil (ELO). Compos Part B-Eng 86168-177
Quiles-Carrillo L, Blanes-Martínez MM, Montanes N, Fenollar O, Torres-Giner S, Balart R (2018) Reactive toughening of injection-molded polylactide pieces using maleinized hemp seed oil. Eur Polymer J 98402–98410
Acknowledgements
This research is a part of the grants PID2023-152869OB-C22, funded by MCIN/AEI/https://doi.org/10.13039/501100011033 and the grant TED2021-131762 A-I00, funded by MCIN/AEI/https://doi.org/10.13039/501100011033 and by the European Union “NextGenerationEU”/PRTR. Authors also thank Generalitat Valenciana-GVA for funding this research through the grant numbers AICO/2021/025 and CIGE/2021/094. J. Gomez-Caturla wants to thank FPU20/01732 grant funded by MCIN/AEI/https://doi.org/10.13039/501100011033 and by ESF Investing in your future.
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature.
Author information
Authors and Affiliations
Contributions
J.J.G-N. did the investigation related to this work, D.G-S. and R.B. wrote the main manuscript text, D.G-G. prepared all Figures, J.G-C. revised and translated the manuscript from spanish to english language.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gazquez-Navarro, J.J., Garcia-Sanoguera, D., Balart, R. et al. A New Use of Polysorbate-Type Nonionic Surfactants as Plasticizers for Highly Flexible Poly(lactide) Formulations. J Polym Environ (2024). https://doi.org/10.1007/s10924-024-03396-1
Accepted:
Published:
DOI: https://doi.org/10.1007/s10924-024-03396-1