Abstract
The novel dioxane-polyether polyurethanes underwent a 12-month outdoor soil burial test to look into how they would degrade in a natural setting. The structure, thermal properties, surface features, and mechanical strength of the polyurethane films were compared. The initial chemical structure and subsequent chemical alterations were identified using FTIR spectroscopy. The polyurethane samples were less thermally stable throughout the duration of the soil burial test, according to TG/DTG curves. According to all findings, polyurethanes containing 1,3-dioxane-5,5-dimethanol exhibit excellent physical characteristics and mild degradation levels after being buried in soil for a year. These polyether urethanes can break down if the rigid domain structure is exposed to moisture and if microorganisms can spread into the polymer matrix. The physical properties, surface features, and degradation of polyether polyurethanes can be improved by varying the molar ratios of the hard segment components and the dioxane derivative structures.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The great qualities that polyurethane elastomers possess enable them to be used in a variety of applications and usage situations. Even while being used, the extreme conditions might cause degradation. The selection of chemical components can considerably enhance the materials structure, characteristics, and resilience against degradation. This leads to the creation of novel polyurethane materials with enhanced structures and features that also exhibit excellent stability [1,2,3].
Due to their widespread use, polyurethanes are particularly challenging to recycle and release hazardous compounds into landfills. In order to aid in degrading polyurethane materials for future recycling, scientists are searching for novel components to incorporate into polyurethane structures [4,5,6,7,8,9,10].
Because polyurethane materials come in a wide range of macromolecular structures, chemical compositions, formulations, and morphologies, analysis of chemical structures is required for efficient waste management [11].
The structure of the most ordered morphological domains, which control moisture accessibility and enzyme diffusion in the polymer matrix, is a major determinant of a polymer’s ability to degrade. Molecular ordering, hydrophobicity, and the ability to pack molecular chains in tight or loose morphological domains are the key factors that determine how accessible the polymer structure is to enzymes and water [12,13,14,15,16,17,18,19,20].
In the case of polyurethanes with the ether structure, no quite efficient biodegradation has been recorded because of the high level of complexity in their polymeric structures [21].
Since polyurethanes have ester and amide groups that are similar to those found in natural macromolecules, they can be broken down by enzymes and taken up by microbial populations in the environment [22,23,24]. According to some theories, polyurethanes’ ester structure makes them vulnerable to hydrolysis by enzymes generated by microorganisms, which eventually causes them to degrade [4, 25,26,27,28].
Polyurethanes made from polyether exhibit resistance to hydrolysis, microbial decay, and corrosion. The urethane segment’s structural modifications may improve its biodegradability [29, 30]. The amount of hard segment contained in polyurethanes has a considerable impact on biodegradation. The larger the hard domain, the more hydrogen bonds there are between the carbonyl groups. This results in limitations on the polymer chain’s movement [31].
In order to increase the variety of features and lessen their environmental impact, there is significant interest in the creation of polyurethane materials that incorporate novel raw materials [32, 33]. Small-molecule removal and alterations in the material’s tensile strength and surface morphology can be used to assess degradation when chemical changes in the polymer structure take place [34].
Polyurethanes made from poly(tetramethylene ether) glycol have excellent physical and dynamic properties in addition to hydrolytic stability [35]. Poly(tetramethylene ether) glycol is a semi-crystalline polymer with low-temperature flexibility and antimicrobial properties [36]. This polyol shows significant elasticity due to the great mobility of the molecular chains, which permit unrestricted rotation around the primary chain links.
The hydroxyl groups of dioxane derivatives with vicinal diols, like 1,4-dioxane-2,3-diols, are helpful for synthesizing polymer structures. Stereoisomeric properties are determined by the dioxane ring’s symmetry. As a result, the trans stereoisomer is more stable than the cis stereoisomer in 1,4-dioxane-2,3-diol [37]. Microorganisms are largely unable to biodegrade 1,4-dioxane because of its cyclic structure and the oxygen incorporated into the carbon chain [38].
Each component of the polyurethane (polyols, diisocyanates and chain extenders) affects biodegradability. Chain extenders have an effect on urethane biodegradation as well as overall performance. There are not many studies on the biodegradability of polyether polyurethanes in soil where the structure of the hard domain was changed to speed up the degradation rates.
The structure and properties of polyurethane materials are significantly altered when dioxane derivatives are added to the matrix ofpolyurethane. Studying the materials’ physical and mechanical characteristics, their hydrophilic surfaces, and the deterioration brought on by composting in soil are all important to assess these effects. Due to its simplicity and resemblance to actual field circumstances, the aerobic soil burial method is frequently used to study the biodegradation of polymeric materials [39,40,41,42,43,44].
This research focuses on the manufacture of novel polyether polyurethanes utilizing dioxane derivatives as well as how they affect the natural degradation process after burial in the soil, where they may end up intentionally or not. It was investigated how the structure and concentration of each type of dioxane derivative affected the performance of the materials and the stability of these polyurethane materials in water. The work provides insight into potential novel polyurethane architectures with controlled stability and desired characteristics.
As a result, extended ether structures in novel polyurethane structures were obtained. Even though they don’t have hydrophilicity, adding dioxane units that boost molecular polarity considerably improves degradation when exposed to the earth’s natural elements. The findings strongly imply that high-performance polyether urethane structures are attainable and that these structures can also exhibit a favorable degradation rate in the environment.
Experimental
Materials
If not otherwise stated, all of the reagents used in this work were analytical grade and used without additional purification. The polyether diol utilized was poly(tetramethylene ether) glycol (PTMEG), which was purchased from Aldrich and has an average Mn of 2000 g/mol and an OH value of 53.4–59.1 mg KOH/g (Sigma-Aldrich Chemie GmbH, Taufkirchen, Germany). Prior to usage, PTMEG was melted and dried for two hours at 80 °C under vacuum (10 mbar).
1,6-Hexamethylene diisocyanate (Mw = 168.19 g/mol, average functionality of around 2, NCO% = 49.94 wt%; HDI) was obtained from Fluka (FlukaChemie AG, Buchs, Switzerland). The chain extenders 1,3-dioxane-5,5-dimethanol (90%, Mw = 148.16 g/mol; DDM) and 1,4-dioxane-2,3-diol (98%, Mw = 120.10 g/mol; D23D) were obtained from Aldrich (Sigma-Aldrich Chemie GmbH, Taufkirchen, Germany). In our laboratory, distilled water was made.
Dioxane-Based Polyurethanes Synthesis
Two series of polyurethanes were produced, each with a distinct structure and percentage of dioxane derivative. Both series of polyurethanes have the same soft segments (PTMEG2000). Their primary structures contain PTMEG, which provides them with excellent hydrolytic cleavage resistance and strong mechanical qualities at low temperatures. So, it is possible to compare how the hard segment structures affect the final characteristics. Table 1 lists the chemical make-up of the synthesized polyurethanes.
As a result, two-stage poly-addition processes were used to create dioxane-based polyurethanes [45]. To create the final polyurethanes, PTMEG2000 was reacted with the required amount of HDI (see Table 1) to obtain a prepolymer, which was reacted with the chain extender while keeping the -NCO and -OH functional groups at the 1:1 molar ratio at this stage.
In thermostatic oil bath and a 250-mL glass reactor equipped with an anchor-shaped stirrer and a Heidolph RZR-2000 mechanical stirrer (both made in Kelheim, Germany), polyether polyurethanes were essentially obtained. Over 20 g of PTMEG2000 that had been heated in the reactor to 80 °C, the necessary amount of HDI was added while being stirred. FTIR spectra were used to keep track of the free NCO concentration throughout the 2-h reaction at 80 °C. Following the addition of the chain extender (a dioxane derivative dispersed in 10 mL of DMF), the reaction was continued for a further two hours at 80 °C with vigorous stirring. Figure 1 shows the dioxane-based polyurethane synthesis scheme.
The polyurethane solutions were cast onto clean glass plates, which were dried under vacuum at 80 °C for 24 h to remove the solvent and create the polymer films. All measurements were made using the resulting films, which ranged in thickness from 0.3 to 0.5 mm.
Hydrolytic Stability Test
A polymer’s capacity to absorb water from the environment can alter its characteristics and the period of usage. The polyurethanes underwent the hydrolytic stability test in accordance with ASTM D 3137. The polyurethane test samples were weighed before being submerged in distilled water for a total of 12 months in ambient settings. Test samples were taken out of the water after 4, 8, and 12 months, and they were then weighed after any extra water had been drained away with filter paper. The following formula was used to calculate hydrolytic stability as a percentage of the sample weight:
Hydrolytic stability(%) = (W1—W2 / W1) 100.
Where W1 is the polyurethane specimen’s original mass and W2 is its mass following the hydrolytic test. No samples demonstrated weight loss or significant swelling.
Biodegradation Test
For 12 months, dumbbell-shaped samples made with dies (ISO 37 type 2) were buried 20 cm deep in neutral garden soils (pH 6.8) near Iasi, Romania (from April 2020 to April 2021). To increase moisture levels and hasten the biodegradation of the films, the soil around the polyurethane samples was watered once a month. Every 4 months, samples were obtained. The polyurethane material was cleaned with distilled water and allowed to dry for 48 h before being characterized.
Characterization Methods
Using a Bruker VERTEX 70 instrument, attenuated total reflection (ATR; diamond crystal at 45°) mode Fourier transform infrared (FT-IR) spectra of the polyurethane films were acquired (Bruker, Karlsruhe, Germany). The spectra were captured with a nominal resolution of 4 cm−1 in the infrared range of 4000–500 cm−1. The stated results were the average of three specimens, and the FT-IR spectra were taken at room temperature.
On STA 449F1 Jupiter (Netzsch, Selb, Germany), thermal gravimetric analyses (TGA) were carried out. Under a nitrogen flow of 50 mL/min and a heating rate of 10 °C/min, measurements were done in the temperature range of 20–700 °C. The samples have a mass of roughly 3–5 mg. There was a 0.2% measurement error margin, on average.
A DSC 200 F3 Maia device (Netzsch, Selb,Germany) was used to perform differential scanning calorimetry (DSC) measurements with a nitrogen flow of 50 mL/min. Using heating and cooling rates of 10 °C/min, a sample with a mass of approximately 10 mg was heated from -100 to 250 °C, cooled at -100 °C, and then heated again from -100 to 250 °C. The DSC has consistently measured enthalpy and heat capacity with an accuracy of 5 and 3%, respectively.
A CAM-101 (KSV Instruments, Helsinki, Finland) contact angle measuring device was used to measure the static contact angle of a liquid on the produced film surface at 25 °C. The contact angle values were measured using two test liquids with various surface tensions: ethylene glycol (99.8% anhydrous, Sigma-Aldrich, Steinheim, Germany) and Milli-Q filtered water (Millipore, ≥ 18.2 MΩ.cm). The droplet size was 1 μL and a Hamilton syringe (Hamilton Company, Reno, Nevada) was employed. The drop was delivered to the surface, and the contact angle was then determined. The average of 10 measurements for each specimen served as a representation of the contact angle value. The overall test results had a standard deviation that was less than 5%.
The tensile characteristics were determined using a Shimadzu EZTest (Shimadzu, Kyoto, Japan), equipped with a 5 kN load cell. The samples were cut into dumbbell shapes (ISO 37 Type 2) without having any surface flaws or burrs along the edges. The tests were conducted with a crosshead speed of 50 mm/min at ambient temperature (23 °C). Five measurements were made for each sample, and average values were obtained with a standard deviation of no more than 5%.
An SEM Quanta 200 with the integrated EDX system was used to analyze changes in the surface morphology of the polyurethanes following an outdoor soil burial test in comparison to the unexposed surface. The Genesis XM 2i EDAX with a Super Ultra-Thin window detector (FEI Company, Hillsboro, USA) was used for this analysis.
Results and Discussion
It is well known that polyurethanes based on polyethers perform better than other materials in terms of hydrolytic stability and fungal resistance [1]. The weight loss of the polymers, changes in the topography and functional groups of the surface, as well as the hydrophobicity and mechanical properties, were used to define the behavior toward degradation. The most frequently reported sites of action for the degrading enzymes are urethane segments, which are more accessible to them when flexible aliphatic diisocyanates are used.
FT-IR Analysis
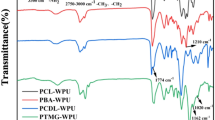
According to Fig. 2, FT-IR spectra were acquired at different test intervals to examine the effects of soil burial degradation on the polyurethane films.
In comparison to the initial samples without exposure to degradation, the study of the polyurethane samples treated to soil burial degradation did not reveal any significant changes in the FT-IR spectra. However, there were indications that the degradation process had begun, particularly after being exposed to soil conditions for a year. Thus, the NH group without hydrogen bonds is responsible for the band in the FT-IR spectrum at 3330 cm−1, which only shows a considerable increase in intensity after 12 months of degradation. Due to the ether structure of both the soft segment and the hard segment, this exhibits a high resistance to the beginning of the degradation process. The spectra of the degraded samples showed a band of average intensity at 1626 cm−1 (DDM2) and 1651 cm−1 (DDM3), which was attributed to the deformation of the NH group [23]. This behavior can be linked to variations in stretching seen in the region between 1038 cm−1 and 1041 cm−1, which is typical of the N-CO–O group in the urethane segment. These changes showed that the DDM structure in the hard domain of the polyurethane films allowed water and fungal enzymes to be absorbed into the urethane segment upon burial in the soil. Due to this, certain inter-urethane linkages were broken and new connections were created in the polymer matrix.
The peak signal that was associated with the C–O–C stretch from the polyurethane DDM3 (1106 cm−1) decreased towards the end of the burial period in the soil. This indicates a microbial attack on the ether groups of the dioxane structure located in the vicinity of the more vulnerable urethane bonds [30].
After 12 months of degradation, all of these peaks exhibit significant signal intensity reductions, indicating that the polyurethane structures produced are susceptible to soil burial degradation. Particularly in the case of D23D polyurethanes, the C-N bond stretching vibration has a peak around 1242–1243 cm−1 (amide III) [46] and gets stronger the longer it is buried in the soil. Amide II exhibits the same behavior between 1535 cm−1 and 1539 cm−1.
The carbonyl group band at 1720 cm−1 (free urethane and urethane bonded to the dioxane ether groups), and particularly at 1704 cm−1 (the hydrogen-bonded urethane groups), showed the most variations. Particularly in the case of polyurethanes with DDM in the construction of hard segments, these peaks shift. This behavior was determined by the greater distances between the carbonyl groups in the polyurethane chain due to the DDM structure. This arrangement increases the accessibility of the degradation factors by reducing the likelihood of interaction between urethane segments.
All of this indicates that the degradation happens around the urethane segments and that a key factor in this process is the structure of the inter-urethane chain extender.
Thermogravimetric Analysis
TG (thermogravimetric curve) and DTG (differential thermogravimetric curve) curves are used to study the thermal decomposition or stability of materials under controlled temperature conditions.
The TG curve is a graphical representation showing the change in mass of a sample as a function of temperature or time under controlled heating conditions. The TG curve provides information about the loss or increase in mass of the sample as it undergoes physical or chemical changes such as decomposition, sublimation, oxidation, reduction, or desorption.
The DTG curve is derived from the TG curve. It represents the rate of mass change with temperature or time. It helps identify the different stages of decomposition or reaction occurring in the sample.
Figure 3 depicts the TG/DTG curves of the polyurethanes before and after degradation in the soil. The D23D’s TG/DTG curves differ from those of the DDM in terms of behavior before and after degradation in the soil.
The TG curves of D23D films revealed a T5% degradation temperature (corresponding to a 5% mass loss) of the above polyurethanes at around 319 °C (D23D1) and 285 °C (D23D2). Furthermore, the maximum decomposition temperatures (Tmax) were around 423 °C (D23D1) and 426 °C (D23D2). The addition of a dioxane derivative to the hard segment structure resulted in a slight decrease in the thermal stability of the analyzed samples. The increase in dioxane derivative amount with the bulkier structure disrupted the physical crosslinking processes, indicating greater mobility of the polyurethane chains and less cohesion between the urethane segments [47].
The T5% value for D23D1 indicates a considerable decrease after being buried in the soil, falling to 248 °C after 8 months (D23D1.2) as opposed to 4 months (D23D1.1; T5% = 282 °C). After being buried in the soil for a year, this temperature (T5% = 325 °C; D23D1.3) returns to the initial sample level (D23D1.0). The T5% values in sample D23D2 rise after 4 months of testing (T5% = 331 °C; D23D2.1), after which they virtually remain constant (272–277 °C for D32D2.2 and D32D2.3, respectively). Contrary to the D23D samples, the T5% values of the DDM samples remain essentially unchanged throughout their 12-month burial in the soil (305–315 °C).
The first step in the thermal degradation process was associated with the thermal decomposition of the urethane bonds. In all dioxane-based polyurethane samples, an increase in T5% values is observed after 12 months of burial in the soil, which shows an increase in the resistance to the thermal decomposition of these polyurethane samples. This could suggest that after a long period of burial in the soil, a reordering takes place in the urethane matrix under the influence of moisture and the mycelium that begins to develop.
The maximum degradation of the DDM polyurethane samples shows two degradation temperature ranges at 338–345 °C (Tmax1) and 414–421 °C (Tmax2), respectively. Therefore, a mass loss of approximately 13.5–16% (Wmax1) corresponds to the first peak of maximum degradation, while a mass decrease of about 55–63% (Wmax2) corresponds to the second peak. The structure of the chain extender, DDM, promotes this phenomenon because it is known that in polyurethane, the hard segments break down first. In addition to volatile chemicals, carbon residues—which in the polymers under study amount to no more than 4.6%—are also produced during the thermal breakdown of polyurethanes (Table 2).
It’s noteworthy to notice that after a year of being buried in the soil, the mass loss in the D23D1 samples during the stage of maximal thermal decomposition goes from 54 (D23D1.0) to 58% (D23D1.3) (Table 2). On the other hand, after being buried in the soil for a year, the mass loss in the DDM3 samples drops from 60.5 (DDM3.0) to 54.6% (DDM3.3). These dioxane-based polyurethanes’ TG/DTG curves show deterioration in thermal stability over a longer period of time (12 months) of soil burial.
DSC Evaluation
The DSC thermograms of the polyurethane films containing the chain extenders DDM and D23D are shown in Fig. 4.
After the second heating, it was observed that the polyurethane films based on DDM showed a single melting temperature (Tm) peak at the maximum temperatures of 20 °C (DDM2) and 22 °C (DDM3) and a single glass transition temperature (Tg) of the soft segments at −76 to −78 °C. As a result of the more rigid D23D structure, it was demonstrated that polyurethanes with a D23D structure have greater Tg values than those with a DDM-based structure.
In the D23D samples, the Tg value decreased with the length of burial in the soil due to a higher degree of deterioration of the hard segments, where the material became weaker. As a result, the soft component enhances their influence in the polyurethane matrix ensemble [48].
Additionally, the D23D samples only exhibit a soft segment melting peak at 25–27 °C, which remains true even after 12 months of earth burial. This demonstrates that burial in the soil has no impact on PTMEG chains and their interactions.
In the DDM samples, the soft segment melting peak (20–22 °C) appears near the soft segment cold crystallization peak at −23 °C (DDM2) and −32 °C (DDM3), respectively. This demonstrates that phase separation can occur to a greater extent in DDM samples than it can in samples made with D23D due to the more elastic structure of DDM. Throughout the whole time of burial in the soil, all of these values are maintained at roughly the same levels. Therefore, the aging in the soil has had little impact on the soft segments of PTMEG in DDM samples.
Polyurethane samples, particularly those that include DDM, exhibit complex behavior with both endothermic and exothermic elements when buried in soil. When the DDM2 samples were first heated, their exothermic components peaked at −23 °C (DDM2.0) and then significantly decreased to −19 °C (DDM2.2), which suggests that the urethane matrix was rearranged to take on a more stable structure. This phenomenon happens in DDM3 samples at about −27 °C. After being buried in the soil for eight months, certain intermolecular urethane bonds break down, causing exothermic activity at 153 °C. This causes rearrangements in an effort to create the most stable structures.
In DDM2.0, the endothermic component that was 90 °C shifts to 48 °C and weakens after being buried in the ground for four months. As more time is spent buried in the soil, this endothermic component disappears. The sharp decline in ordering within the hard domains was the cause of this phenomenon [16].
It is important to notes that neither in the original sample (DDM2.0) nor after 12 months of burial in the soil does a crystallization exotherm develop in the DDM2 sample during the comparable cooling scan. The crystallization exotherm in sample DDM3 happens at −34 °C. The crystallization exotherm in sample D23D1 occurs at −36 °C (D23D1.0), and it drops to −31 °C after 12 months of soil burial. After 12 months of burial in the soil, the temperature of the crystallization exotherm in sample D23D2 decreases from 22 °C (D23D2.0) to 13 °C (D23D2.3).
The entire process of change throughout the period of burial in the soil is driven by the behavior of the connections of the molecule segments, particularly hydrogen bonds, which are essential to preserving the structure of urethane matrices.All of these are impacted by the quantity and composition of flexible segments in the hard domains [7, 49]. As a result of the greater intermolecular ordering and bonding in the hard domains, moisture and microbe accessibility are constrained in the region where urethane polar groups are present.
According to all the data, the urethane group structure prevented adequate urethane matrix packing, which is why the polyurethanes containing dioxane components mainly broke down in the hard segments.
Mechanical Properties
The tensile strength of the polyurethane dumbbells buried in the soil for various amounts of time (4, 8, and 12 months) was evaluated and compared to the non-degraded samples in order to assess the degree of degradation. Depending on the structure of the dioxane derivative between the urethane groups, the tensile strength of the polyurethane samples buried in the soil exhibited various behaviors (Fig. 5).
The stress versus strain of the polyurethanes acquired before (D23Dx.0; DDMx.0) and after degradation by burial in soil for 4 months (D23Dx.1; DDMx.1), 8 months (D23Dx.2; DDMx.2), and 12 months (D23Dx.3; DDMx.3).
As a result, after 12 months of degradation, the tensile strength of the D23D1 sample has decreased constantly, from 18 MPa (D23D1.0) to 6 MPa (D23D1.3). Additionally, the break elongation falls from 750 (D23D1.0) to 600% (D23D1.3). At greater D23D content, the mechanical characteristics of the polyurethane structure drop to a maximum tensile strength of 7.4 MPa (D23D2.0). The increase in D23D content, whose more rigid structure resulted in poorer molecular chain ordering, which weakened the polymer matrix and reduced molecular chains interaction.
After being buried in the soil for 8 months, the tensile strength of the DDM2 samples only slightly decreased (from 14 MPa (DDM2.0) to 13 MPa (DDM2.2)). After a year of degradation, when the tensile strength approaches 11 MPa (DDM2.3) and the elongation at break drops from 1260 (DDM2.0) to 1080% (DDM2.3), a more noticeable decline is seen.
The tensile strength of the samples with a higher DDM content increased from 14 MPa (DDM3.0) to 20 MPa (DDM3.1) after being buried in the soil for 4 months, while the elongation at break fell from 1200 (DDM3.0) to 1000% (DDM3.1). Tensile strength significantly declines and reaches 2 MPa after a year of being buried in the soil (DDM3.3).
Changes in the tensile strength and elongation at break of polyether polyurethanes that contain dioxane derivatives are signs of the beginning of physical deterioration.
Contact Angle Measurement
The dynamics of the surface energy resulting from the measurement of the contact angle with water is used to assess changes in the distribution of functional groups on the surface of polyurethane films during degradation. The wettability of surfaces can be improved by increasing their hydrophilicity, which encourages microorganisms’ adhesion to the polymer surface and subsequent breakdown [34].
The structure of the hard domain of the dioxane-based polyurethane chains has an impact on the migration of polar groups to the surface of the polymer, enhancing its hydrophilicity. The water contact angle for D23D1.0 was the smallest (83°). It decreases to 79° (D23D1.1) after 4 months of being buried in the ground, and stays at that level for the remaining 12 months of the degradation test (Table 3).
D23D2 polyurethanes include a higher amount of D23D and have a substantially higher contact angle with water (96°) due to the urethane segments enhanced stiffness, which limits the migration of polar groups to the polymer surface. The contact angle with water reduces to 84° after 4 months of burial in the soil. Throughout the degradation test, this value remains the same.
In contrast, the DDM samples had high beginning values for the contact angle with water, which are around 96°. The contact angle dropped to 83° (DDM2.1) and 81° (DDM3), respectively, after 4 and 12 months of burial in the soil. The DDM structure allowed for intra- and intermolecular rearrangement after 8 months in the soil, resulting in contact angles of 94°. A decrease in the water contact angle values was seen at the end of the test period, which was more obvious in the samples with a greater level of DDM (DDM3.3; 79°).
The affinity to water of the polyurethanes obtained with D23D can also be seen from the higher values of the polar component of the surface energy (γpsv) which for D23D1.0 was 13.6 mN/m and increased to 17.5 mN/m after 4 months of burial in the soil (Table 4). A large increase was also observed in polyurethanes containing DDM after 4 weeks of burial in the soil. Thus, DDM2 increased from 2 mN/m to 12 mN/m, while DDM3 increased from 1.59 mN/m to 18.6 mN/m. This was due to the molecular reorganization allowed by the DDM structure and the exposure of several polar groups on the surface capable of forming hydrogen bonds with water.
The higher values of the polar component of surface energy (γpsv), which for D23D1.0 was 13.6 mN/m and increased to 17.5 mN/m after 4 months of burial in the soil, further indicate the polyurethanes obtained with D23D’s attraction to water (Table 4). After being buried in the soil for 4 weeks, DDM-containing polyurethanes also showed a significant rise in the polar component of the surface energy (γpsv). The polar component of the surface energy of the DDM2 increased from 2 mN/m to 12 mN/m, while that of the DDM3 grew from 1.59 mN/m to 18.6 mN/m. The DDM structure’s ability to facilitate molecular rearrangement and the surface exposure of many polar groups capable of forming hydrogen bonds with water were the causes of this.
Evaluation of the Hydrolytic Stability
The urethane group is the primary target of the hydrolytic breakdown in the case of polyurethane based on polyether polyol. The degree of swelling has a significant impact on many other aspects, including the rate of hydrolytic deterioration. Figure 6 shows the dynamics of how much the polyurethane samples swelled after being submerged in water for a year.
When submerged in water, all the polyurethane samples behaved pretty similarly. None of the recently created dioxane-based polyurethanes exhibit any appreciable swelling. However, the D23D polyurethanes showed a slight swelling. The more stiff structure of D23D, which discourages a good packing of the urethane matrix, is to blame for this behavior. Increased water absorption occurs as a result of easier water infiltration into the polymer matrix caused by the larger space between the disordered polyurethane chains.
Dioxane-based polyurethane samples displayed relatively little swelling, up to 2–3%. After 4 months of submersion in water, DDM3 experiences the highest degree of swelling (9.5%), which, after 12 months, returns to the initial amount of 2–3%. The existence of ether linkages in both the soft and hard segments of these polyurethanes, which are resistant to hydrolytic attack, was thought to be the cause of the weak swelling of these materials.
SEM Analysis
The polymer surface was altered after being buried in the soil for varied amounts of time, and this was demonstrated using SEM. The topographic differences at the polymer’s surface can be clearly seen using SEM. Visible changes in polymer morphology might be indicated by holes and cracks, increased roughness, or the adhesion of microorganisms as a result of surface degradation. Figure 7 displays morphological images of polyurethane films created from dioxane derivatives before and after being buried in the soil for 4, 8, and 12 months.
It is evident that the film surface has started to deteriorate after being buried in the ground for a period of one year. Sample D23D1.3 shows deep, branching fissures after being buried for 12 months, along with fragments that are detached from its surface. The deterioration of the film surface is particularly obvious in this polyurethane structure. These findings concur with the findings of the mechanical measures, whose values gradually dropped after being buried in the soil. These degradations take place in hard segment domains containing D23D rigid structures. The fact that D23D2.3 and DDM2.3 film surfaces have a crushed appearance suggests that these polyurethanes require more time to degrade.
DDM reduced the permeability of the film to water by increasing the possibility of molecular packing and ordering in the urethane structure.
Conclusions
The synthesis and evaluation of polyurethanes that contain dioxane derivatives were investigated in this work. According to TG/DTG measurements, polyurethanes lost some of their thermal stability after degrading through soil burial. After being buried in the soil, urethane bond-related changes were seen in the FT-IR spectrum. Adding segments of dioxane derivative to polyurethane chains reduces the dispersion component and favors the polar component of the surface energy, following degradation by burial in the soil. All of the results point to the area of the urethane segments as the primary location for degradation. Particularly for the D23D1 polyurethane sample, degradation was clearly confirmed. Although polyurethanes are generally known for their resistance to degradation, the incorporation of dioxane derivative segments may improve their biodegradability.
In conclusion, the incorporation of dioxane derivative segments into polyether polyurethanes offers a promising way to improve both the physical properties and the environmental characteristics of these materials.
Data Availability
The data presented in this study are available on request from the corresponding author.
References
Xie F, Zhang T, Bryant P, Kurusingal V, Colwell JM, Laycock B (2019) Degradation and stabilization of polyurethane elastomers. Prog Polym Sci 90:211–268
Kaikade DS, Sabnis AS (2023) Recent advances in polyurethane coatings and adhesives derived from vegetable oil-based polyols. J Polym Environ 31:4583–4605
Magana I, Lopez R, Enriquez-Medrano FJ, Kumar S, Aguilar-Sanchez A, Handa R, Diaz de Leon R, Valencia L (2022) Bioelastomers: current state of development. J Mater Chem A 10:5019–5043
Howard GT (2002) Biodegradation of polyurethane: a review IntBiodeteriorBiodegrad 49:245–252
Cardenas Espinosa MJ, Colina Blanco A, Schmidgall T, Atanasoff-Kardjalieff AK, Kappelmeyer U, Tischler D, Pieper DH, Heipieper HJ, Eberlein C (2020) Toward biorecycling: isolation of a soil bacterium that grows on a polyurethane oligomer and monomer. Front Microbiol 11:404
Di Bisceglie F, Quartinello F, Vielnascher R, Guebitz GM, Pellis A (2022) Cutinase-catalyzed polyester-polyurethane degradation: elucidation of the hydrolysis mechanism. Polymers 14:411
Lopes RVV, Loureiro NPD, Quirino RL, Gomes ACM, Pezzin APT, Manzur LP, dos Santos ML, Sales MJA (2022) Biodegradation study of polyurethanes from linseed and passion fruit oils. Coatings 12:617
Delidovich I, Hausoul PJC, Deng L, Pfutzenreuter R, Rose M, Palkovits R (2016) Alternative monomers based on lignocellulose and their use for polymer production. Chem Rev 116:1540–1599
Brannigan RP, Walder A, Dove AP (2019) Application of functional diols derived from pentaerythritol as chain extenders in the synthesis of novel thermoplastic polyesterurethane elastomers. Polym Chem 10:5236–5241
Tiso T, Winter B, Wei R, Hee J, de Witt J, Wierckx N, Quicker P, Bornscheuer UT, Bardow A, Nogales J, Blank LM (2022) The metabolic potential of plastics as biotechnological carbon sources – review and targets for the future. Metab Eng 71:77–98
Magnin A, Pollet E, Phalip V, Averous L (2020) Evaluation of biological degradation of polyurethanes. Biotechnol Adv 39:107457
Zambrano MC, Pawlak JJ, Venditti RA (2020) Effects of chemical and morphological structure on biodegradability of fibers, fabrics, and other polymeric materials. BioResources 15:9786–9833
Mohanan N, Montazer Z, Sharma PK, Levin DB (2020) Microbial and enzymatic degradation of synthetic plastics. Front Microbiol 11:580709
Varyan I, Tyubaeva P, Kolesnikova N, Popov A (2022) Biodegradable polymer materials based on polyethylene and natural rubber: acquiring, investigation, properties. Polymers 14:2457
Tai NL, Adhikari R, Shanks R, Adhikari B (2019) Aerobic biodegradation of starch–polyurethane flexible films under soil burial condition: changes in physical structure and chemical composition. Int Biodeterior Biodegrad 145:104793
Laycock B, Nikolic M, Colwell JM, Gauthier E, Halley P, Bottle S, George G (2017) Lifetime prediction of biodegradable polymers. Prog Polym Sci 71:144–189
Beydoun K, Klankermayer J (2020) Efficientplastic waste recycling to value-added products by integrated biomass processing. Chem Sus Chem 13:488–492
Saleemi MA, Lim V (2022) Overview of antimicrobial polyurethane-based nanocomposite materialsand associated signalling pathways. Eur Polym J 167:111087
Srikanth M, Sandeep TSRS, Sucharitha K, Godi S (2022) Biodegradation of plastic polymers by fungi: a brief review. Bioresour Bioprocess 9:42
Beckerab G, Wurm FR (2018) Functional biodegradable polymers via ring-opening polymerization of monomers without protective groups. Chem Soc Rev 47:7739–7782
Liu J, He J, Xue R, Xu B, Qian X, Xin F, Blank LM, Zhou J, Wei R, Dong W, Jiang M (2021) Biodegradation and up-cycling of polyurethanes: progress, challenges, and prospects. Biotechnol Adv 48:107730
Cosgrove L, McGeechan PL, Handley PS, Robson GD (2010) Effect of biostimulation and bioaugmentation on degradation of polyurethane buried in soil. Appl Environ Microbiol 76:810–819
Khan S, Nadir S, Shah ZU, Shah AA, Karunarathna SC, Xu J, Khan A, Munir S, Hasan F (2017) Biodegradation of polyester polyurethane by aspergillus tubingensis. Environ Pollut 225:469–480
Shah Z, Gulzar M, Hasan F, Shah AA (2016) Degradation of polyester polyurethane by an indigenously developed consortium of pseudomonas and bacillus species isolated from soil. Polym Degrad Stab 134:349–356
Cosgrove L, McGeechan PL, Robson GD, Handley PS (2007) Fungal communities associated with degradation of polyester polyurethane in soil. Appl Environ Microbiol 73:5817–5824
Shah AA, Hasan F, Akhter JI, Hameed A, Ahmed S (2008) Degradation of polyurethane by novel bacterial consortium isolated from soil. Ann Microbiol 58:381–386
Osman M, Satti SM, Luqman A, Hasan F, Shah Z, Shah AA (2018) Degradation of polyester polyurethane by Aspergillus sp. strainS45 isolated from soil. J Polym Environ 26:301–310
Sahoo S, Kalita H, Mohanty S, Nayak SK (2018) Degradation study of biobased polyester–polyurethane and its nanocomposite under natural soil burial, UV radiationand hydrolytic-saltwater circumstances. J Polym Environ 26:1528–1539
Mahajana N, Gupta P (2015) New insights into the microbial degradation of polyurethanes. RSC Adv 5:41839–41854
Gaytan I, Sanchez-Reyes A, Burelo M, Vargas-Suarez M, Liachko I, Press M, Sullivan S, Cruz-Gomez MJ, Loza-Tavera H (2020) Degradation of recalcitrant polyurethane and xenobiotic additives by a selected landfill microbial community and its biodegradative potential revealed by proximity ligation-based metagenomic analysis. Front Microbiol 10:2986
Oprea S, Potolinca VO (2023) Water-dispersible polyurethanes obtained by the controlled alternation of the segments of poly(propylene glycol), poly(ethylene glycol) and urethane. J Polym Environ 31:3754–3767
Gunawan NR, Tessman M, Schreiman AC, Simkovsky R, Samoylov AA, Neelakantan NK, Bemis TA, Burkart MD, Pomeroy RS, Mayfield SP (2020) Rapid biodegradation of renewable polyurethane foams with identification of associated microorganisms and decomposition products. BioresourTechnol Rep 11:100513
Singh P, Kumar KD, Kumar R (2022) Degradation of polyfurfuryl alcohol-based biopolymer by soil-burial and photo-degradation methods. J Polym Environ 30:1920–1931
Chamas A, Moon H, Zheng J, Qiu Y, Tabassum T, Jang JH, Abu-Omar M, Scott SL, Suh S (2020) Degradation rates of plastics in the environment. ACS Sustain Chem Eng 8:3494–3511
Wang Y, Song J, Tian Q, Song N, Liang S, Tian C, Qiang X, Lei Y, Chen K, Almasy L (2023) Understanding water absorption effect on molecular dynamics, microstructures and relaxation behavior of segmented polyurethane elastomers. Polym Degrad Stab 214:110415
Isikgor FH, Becer CR (2015) Lignocellulosic biomass: a sustainable platform for the production of bio-based chemicals and polymers Polym Chem 6:4497–4559
Ayras P (1978) On the stereochemistry of 1,4-diheterocyclanes. IV-carbon-13 NMR spectra and structural properties of 1,4-dioxane-2,3-diols and their methyl-substituted derivatives. Org Magn Reson 11:152–156
Adams CD, Scanian PA, Secrlst ND (1994) Oxidation and biodegradability enhancement of 1,4-dioxane using hydrogen peroxide and ozone. Environ Sci Technol 28:1812–1818
Oprea S, Oprea V (2016) Biodegradation of crosslinked polyurethane acrylates/guar gum composites under natural soil burial conditions. Polymers 16:277–286
Oprea S (2015) Effects of introducing crude and modified soybean oil into polyurethane structures on the soil-burial biodegradation process. Polym Plast Technol Eng 54:342–3497
Faccia PA, Pardini FM, Agnello AC, Amalvy JI, Del Panno MT (2021) Degradability of poly(ether-urethanes) and poly(ether-urethane)/acrylic hybrids by bacterial consortia of soil. Int Biodeterior Biodegrad 160:105205
Zuliani A, Rapisarda M, Chelazzi D, Baglioni P, Rizzarelli P (2022) Synthesis, characterization, and soil burial degradation of biobased polyurethanes. Polymers 14:4948
Wu K-Y, Yang T-X, Yang M, Wu J-Q, Li X, Chen X-D, Tang L, Yang X-Y (2023) Preliminary identification of soil fungi for the degradationof polyurethane film. Arch Microbiol 205:145
Olivito F, Jagdale P, Oza G (2023) Synthesis and biodegradation testof a new polyether polyurethane foam produced from PEG 400, L-lysine ethyl ester diisocyanate(L-LDI) and bis-hydroxymethyl furan (BHMF). Toxics 11:698
Oprea S, Potolinca VO (2022) On improving the physical properties of poly (urethane urea)s by the inclusion of aromatic amines connected through long aliphatic chains in the hard domain. Eur Polym J 166:111035
Wang Y, Song H, Ge H, Wang J, Wang Y, Jia S, Deng T, Hou X (2018) Controllable degradation of polyurethane elastomer via selective cleavage of C-O and C-N bonds. J Clean Prod 176:873–879
Xu WJ, Wang JJ, Zhang SY, Sun J, Qin CX, Dai LX (2018) Tuning chain extender structure to prepare highperformancethermoplastic polyurethaneelastomers. RSC Adv 8:20701–20711
Fuensanta M, Martin-Martinez JM (2020) Viscoelastic and adhesion properties of new poly(ether-urethane)pressure-sensitive adhesives. Front Mech Eng 6:34
Bialkowska A, Mucha K, Przybylek M, Bakar M (2018) Effect of hard segments content on the properties, structure and biodegradation of nonisocyanate polyurethane. Polym Polym Compos 26:423–430
Author information
Authors and Affiliations
Contributions
All authors have equal contribution to the paper. The corresponding author is responsible for ensuring that the descriptions are accurate and agreed by all authors.
Corresponding author
Ethics declarations
Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Oprea, S., Potolinca, V.O. & Gradinaru, L.M. The Impact of Adding Dioxane Derivatives to Polyurethane Structures on their Performance and Degradation in the Environment. J Polym Environ (2024). https://doi.org/10.1007/s10924-024-03288-4
Accepted:
Published:
DOI: https://doi.org/10.1007/s10924-024-03288-4