Abstract
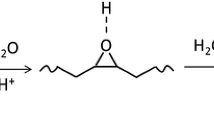
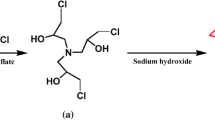
Adhesives are used to bond two surfaces together and thus find extensive applications in industry and everyday life. Most of the commercial adhesives are manufactured using formaldehyde which makes them toxic to humans and the environment. In addition, the low bonding strength of adhesives in humid environments and underwater needs to be improved for their wider applications. This work prepared and tested a green adhesive using soybean oil. The bio-based non-isocyanate polyurethane (NIPU) adhesives were synthesized by reacting carbonated soybean oil (CSBO) with different amines such as 1,2-ethylenediamine (EDA), 1,4-butylenediamine (BDA), and 1,6-hexamethylenediamine (HDA) in a solvent and catalyst-free approach. The effect of amines on the bonding characteristics such as swelling percentage, adhesion strength, and thermal properties of the adhesives were explored. The dry lap shear strength of the adhesives prepared using EDA, BDA, and HDA showed a bonding strength of 6.23, 8.26, and 7.22 MPa, respectively. Interestingly, the wet lap shear strength of HDA-based adhesive was observed to be around 7.49 MPa while it was 5.8 MPa for BDA and 2.8 MPa for EDA. The increasing chain length of diamines exhibits a decreasing trend in swelling percentage in different solvents which suggests a stronger crosslinking of NIPU adhesives. However, HDA has lower tensile strength than BDA due to its brittleness nature. The high bonding strength of soybean oil-based adhesives suggests that these green adhesives could be used to produce high-quality interior-grade plywood.








Similar content being viewed by others
Data Availability
No datasets were generated or analysed during the current study.
References
Pilar M, Blasco C, Silvestre CR (2022) Sustainable Reactive Polyurethane Hot Melt Adhesives Based on Vegetable Polyols for Footwear Industry
Wang YZ, Hsu YC, Wu RR, Kao HM (2003) Synthesis and structure properties of polyurethane based conducting copolymer I. 13 C NMR analysis. Synth Met 132:151–160
Khatoon H, Ahmad S (2017) A review on conducting polymer reinforced polyurethane composites. J Ind Eng Chem 53:1–22
Blattmann H, Fleischer M, Bähr M, Mülhaupt R (2014) Isocyanate- and phosgene-free routes to polyfunctional cyclic carbonates and green polyurethanes by fixation of carbon dioxide, Macromol. Rapid Commun 35:1238–1254
Panchal U, Chaudhary ML, Patel P, Patel J, Gupta RK (2024) Soybean-Based Bio-Adhesives : Role of Diamine on the Adhesive Properties
Kote P, Asare M, Chaudhary S, de Souza FM, Patel P, Gupta RK (2023) Synergistic Effect of P and N-Based Flame Retardants on Bio-Based Polyurethane Foams, in: Polyurethanes Prep. Prop. Appl. Vol. 2 Adv. Appl., American Chemical Society, : pp. 5–71
Acuña P, Zhang J, Yin G-Z, Liu X-Q, Wang D-Y (2021) Bio-based rigid polyurethane foam from castor oil with excellent flame retardancy and high insulation capacity via cooperation with carbon-based materials. J Mater Sci 56:2684–2701
Poussard L, Mariage J, Grignard B, Detrembleur C, Jéroîme C, Calberg C, Heinrichs B, De Winter J, Gerbaux P, Raquez JM, Bonnaud L, Dubois P (2016) Non-isocyanate polyurethanes from carbonated soybean oil using Monomeric or Oligomeric diamines to achieve thermosets or thermoplastics. Macromolecules 49:2162–2171
Beniah G, Fortman DJ, Heath WH, Dichtel WR, Torkelson JM (2017) Non-isocyanate polyurethane Thermoplastic Elastomer: Amide-based chain extender yields enhanced nanophase separation and Properties in Polyhydroxyurethane. Macromolecules 50:4425–4434
Bossion A, Jones GO, Taton D, Mecerreyes D, Hedrick JL, Ong ZY, Yang YY, Sardon H (2017) Non-isocyanate polyurethane soft nanoparticles obtained by surfactant-assisted interfacial polymerization. Langmuir 33:1959–1968
Jazi ME, Al-Mohanna T, Aghabozorgi F (2016) Synthesis and applications of isocyanate free polyurethane materials. Glob J Sci Front Res B Chem 16:1–20
Cornille A, Michaud G, Simon F, Fouquay S, Auvergne R, Boutevin B, Caillol S (2016) Promising mechanical and adhesive properties of isocyanate-free poly(hydroxyurethane). Eur Polym J 84:404–420
Khatoon H, Iqbal S, Irfan M, Darda A, Rawat NK (2021) A review on the production, properties and applications of non-isocyanate polyurethane: a greener perspective. Prog Org Coat 154:106124
Patel P, De Souza FM, Gupta RK (2024) Study of soybean oil-based non-isocyanate polyurethane films. via a Solvent and Catalyst-Free Approach
Doley S, Dolui SK (2018) Solvent and catalyst-free synthesis of sunflower oil based polyurethane through non-isocyanate route and its coatings properties. Eur Polym J 102:161–168
Polyurethane N, Zhang B, Yang X, Lin X, Shang H, Liu Q, Wang H, Liu S (2023) High-Strength, Self-Healing, Recyclable, and Catalyst-Free Bio-Based Non-Isocyanate Polyurethane
Maisonneuve L, Lebarbé T, Grau E, Cramail H (2013) Structure-properties relationship of fatty acid-based thermoplastics as synthetic polymer mimics. Polym Chem 4:5472–5517
Maisonneuve L, Lamarzelle O, Rix E, Grau E, Cramail H (2015) Isocyanate-free routes to polyurethanes and poly(hydroxy urethane)s. Chem Rev 115:12407–12439
Foltran S, Maisonneuve L, Cloutet E, Gadenne B, Alfos C, Tassaing T, Cramail H (2012) Solubility in CO 2 and swelling studies by in situ IR spectroscopy of vegetable-based epoxidized oils as polyurethane precursors. Polym Chem 3:525–532
Maisonneuve L, More AS, Foltran S, Alfos C, Robert F, Landais Y, Tassaing T, Grau E, Cramail H (2014) Novel green fatty acid-based bis-cyclic carbonates for the synthesis of isocyanate-free poly(hydroxyurethane amide)s. RSC Adv 4:25795–25803
Maisonneuve L, Wirotius AL, Alfos C, Grau E, Cramail H (2014) Fatty acid-based (bis) 6-membered cyclic carbonates as efficient isocyanate free poly(hydroxyurethane) precursors. Polym Chem 5:6142–6147
Ochiai B, Inoue S, Endo T (2005) One-pot non-isocyanate synthesis of polyurethanes from bisepoxide, carbon dioxide, and diamine. J Polym Sci Part Polym Chem 43:6613–6618
Paddock RL, Nguyen ST (2001) Chemical CO2 fixation: CR(III) salen complexes as highly efficient catalysts for the coupling of CO2 and epoxides. J Am Chem Soc 123:11498–11499
Yang Z, Peng H, Wang W, Liu T (2010) Crystallization behavior of poly(ε-caprolactone)/layered double hydroxide nanocomposites. J Appl Polym Sci 116:2658–2667
Tamami B, Sohn S, Wilkes GL (2004) Incorporation of carbon dioxide into soybean oil and subsequent preparation and studies of nonisocyanate polyurethane networks. J Appl Polym Sci 92:883–891
Bähr M, Mülhaupt R (2012) Linseed and soybean oil-based polyurethanes prepared via the non-isocyanate route and catalytic carbon dioxide conversion. Green Chem 14:483–489
Javni I, Hong DP, Petrovic̈ ZS (2013) Polyurethanes from soybean oil, aromatic, and cycloaliphatic diamines by nonisocyanate route. J Appl Polym Sci 128:566–571
Asare M.A., Kote P, Chaudhary S, de Souza F.M., Gupta R.K. (2022) Sunflower Oil as a renewable resource for polyurethane foams: effects of Flame-Retardants. Polym (Basel) 14:5282
Teo L, Chen C, Kuo J (1997) Fourier Transform Infrared Spectroscopy Study on effects of temperature on Hydrogen Bonding in Amine-containing polyurethanes and poly (urethane - urea) s, 9297 1793–1799
Panchireddy S, Grignard B, Thomassin J-M, Jerome C, Detrembleur C (2018) Bio-based poly(hydroxyurethane) glues for metal substrates. Polym Chem 9:2650–2659
Bähr M, Bitto A, Mülhaupt R (2012) Cyclic limonene dicarbonate as a new monomer for non-isocyanate oligo- and polyurethanes (NIPU) based upon terpenes. Green Chem 14:1447–1454
Nielsen LE (2008) Journal of Macromolecular Science, Part C : polymer reviews cross-linking – effect on Physical properties of polymers. J Macromol Sci Part C Polym Rev 3:69–103
Gomez-Lopez A, Grignard B, Calvo I, Detrembleur C, Sardon H (2020) Monocomponent Non-isocyanate polyurethane adhesives based on a Sol-Gel process. ACS Appl Polym Mater 2:1839–1847
Yang H, Du G, Li Z, Ran X, Zhou X, Li T, Gao W, Li J, Lei H, Yang L (2021) Superstrong Adhesive of Isocyanate-Free Polyurea with a branched structure. ACS Appl Polym Mater 3:1638–1651
Kawalerczyk J, Dziurka D, Mirski R, Trociński A (2019) Flour fillers with urea-formaldehyde resin in plywood. BioResources 14:6727–6735
Peng J, Liu F, Feng F, Feng X, Cui J (2023) Enhancing environmentally friendly tannin Adhesive for Plywood through Hyperbranched Polyamide. ACS Sustain Chem Eng 11:13805–13811
Pichelin F, Kamoun C, Pizzi A (1999) Hexamine hardener behaviour: effects on wood glueing, tannin and other wood adhesives. Holz Als Roh - Und Werkst 57:305–307
Pizzi A, Cameron FA (1981) Decrease of pressing temperature and adhesive content by metallic ion catalysis in tannin-bonded particleboard, Holz Als Roh- Und Werkst. 39 463–467
Li K, Geng X, Simonsen J, Karchesy J (2004) Novel wood adhesives from condensed tannins and polyethylenimine. Int J Adhes Adhes 24:327–333
Chen X, Pizzi A, Fredon E (2021) C. Gerardin, curing temperature polyurethane (NIPU) wood adhesives : Preparation and properties evaluation Abstract :, 1–28
Xi X, Pizzi A, Frihart CR, Lorenz L, Gerardin C (2020) Tannin plywood bioadhesives with non-volatile aldehydes generation by specific oxidation of mono- and disaccharides. Int J Adhes Adhes 98
Dong T, Dheressa E, Wiatrowski M, Pereira AP, Zeller A, Laurens LML, Pienkos PT (2021) Assessment of Plant and Microalgal Oil-Derived Nonisocyanate polyurethane products for potential commercialization, ACS sustain. Chem Eng 9:12858–12869
He X, Xu X, Wan Q, Bo G, Yan Y (2019) Solvent- and Catalyst-free synthesis, hybridization and characterization of Biobased Nonisocyanate polyurethane (NIPU), polymers (Basel). 11
Mó O, Yáñez M, Eckert-Maksić M, Maksić ZB, Alkorta I, Elguero J (2005) Periodic trends in Bond Dissociation energies. A theoretical study. J Phys Chem A 109:4359–4365
Aung MM, Yaakob Z, Kamarudin S, Abdullah LC (2014) Synthesis and characterization of Jatropha (Jatropha curcas L.) oil-based polyurethane wood adhesive. Ind Crops Prod 60:177–185
Ang KP, Lee CS, Cheng SF, Chuah CH (2014) Synthesis of palm oil-based polyester polyol for polyurethane adhesive production. J Appl Polym Sci 131:1–8
Tenorio-Alfonso A, Sánchez MC, Franco JM (2017) Preparation, characterization and mechanical properties of bio-based polyurethane adhesives from isocyanate-functionalized cellulose acetate and castor oil for bonding wood. Polym (Basel) 9:1–14
Vieira FR, Gama N, Magina S, Barros-Timmons A, Evtuguin DV, Pinto PCOR (2022) Polyurethane Adhesives Based on Oxyalkylated Kraft Lignin, Polymers (Basel). 14
Author information
Authors and Affiliations
Contributions
PP, RP, MLC and NC performed all the experiments, analyzed the data, and wrote the first draft. RKG conceived and supervised the project, and finalized the draft.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Patel, P., Patel, R., Chaudhary, M.L. et al. High-Performance Bio-Based Non-Isocyanate Polyurethane Adhesive: A Solvent and Catalyst-Free Synthesis Approach. J Polym Environ (2024). https://doi.org/10.1007/s10924-024-03277-7
Accepted:
Published:
DOI: https://doi.org/10.1007/s10924-024-03277-7