Abstract
In this study, we conducted the synthesis of a composite material by grafting an acrylonitrile-co-styrene (AN-co-St) polymer into sodium alginate and incorporating CuO (copper oxide) and TiO2 (titanium dioxide) nanoparticles. The primary objective was to investigate the potential anticancer and antioxidant activities of the composite material. First, CuO and TiO2 nanoparticles were synthesized and characterized for their size, morphology, and surface properties. Subsequently, these nanoparticles were integrated into the sodium alginate matrix, which had been grafted with the AN-co-St polymer, resulting in the formation of the composite material. To confirm successful nanoparticle incorporation and assess the structural integrity of the composite, various techniques such as X-ray diffraction analysis (XRD), scanning electron microscopy-energy dispersive X-ray analysis (SEM-EDX), Fourier-transform infrared spectroscopy (FTIR), and thermogravimetric analysis (TGA) were employed. The composite material’s anticancer and antioxidant activities were then evaluated. In vitro cell viability assays using the HepG-2 cell line were performed to assess potential cytotoxic effects, while antioxidant (DPPH) assays were conducted to determine the composite’s ability to scavenge free radicals and protect against oxidative stress. Preliminary results indicate that the composite material demonstrated promising anticancer and antioxidant activities. The presence of CuO and TiO2 nanoparticles within the composite contributed to these effects, as these nanoparticles are known to possess anticancer and antioxidant properties. Furthermore, the grafting of the AN-co-St polymer into sodium alginate enhanced the overall performance and stability of the composite material.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hepatocellular carcinoma (HCC) is the most common type of primary liver cancer and is a significant global health concern. It arises from hepatocytes, the main functional cells of the liver. HCC is characterized by rapid tumour growth and has a high mortality rate [1]. The development of HCC is often attributed to underlying chronic liver diseases, such as hepatitis B virus (HBV) or hepatitis C virus (HCV) infection, alcoholic liver disease, non-alcoholic fatty liver disease (NAFLD), and cirrhosis. These conditions lead to liver inflammation, cell damage, and the formation of fibrous scar tissue, creating an environment conducive to the development of cancerous cells [2]. The incidence of HCC varies across different regions of the world, with a higher prevalence in regions where chronic viral hepatitis is endemic, such as East Asia and sub-Saharan Africa [3, 4]. However, the incidence of HCC is also rising in Western countries due to the increasing prevalence of NAFLD associated with obesity and metabolic syndrome [5, 6]. The diagnosis of HCC typically involves a combination of imaging techniques, such as ultrasound, computed tomography (CT) scan, and magnetic resonance imaging (MRI), along with blood tests to detect elevated levels of tumour markers, such as alpha-fetoprotein (AFP) [7, 8]. Confirmation of the diagnosis often requires a liver biopsy [9, 10]. Treatment options for HCC depend on the stage of the disease, the underlying liver function, and the overall health of the patient. They may include surgical resection, liver transplantation, radiofrequency ablation, transarterial chemoembolization, targeted therapies, and systemic chemotherapy [11, 12]. However, the prognosis for HCC remains generally poor, mainly due to late-stage diagnosis and limited treatment options for advanced disease [13]. Grafted sodium alginate polymers find applications in a wide range of fields, including drug delivery systems, tissue engineering, coatings, and food science [14, 15]. The modified properties of grafted sodium alginate make it a versatile material with enhanced functionality and tailored characteristics [16]. Grafting polymers into sodium alginate is a process that involves covalently attaching polymer chains onto the backbone of sodium alginate, a natural polysaccharide derived from brown seaweed [17]. This technique is commonly used to modify the properties of sodium alginate and enhance its functionality for various applications [18]. The grafting process typically involves two main steps: activation of the sodium alginate backbone and subsequent polymerization or attachment of the polymer chains. Different methods can be employed for each step, depending on the desired properties and application requirements [16, 19,20,21]. The grafting of polymers into sodium alginate can introduce various modifications to the material, such as improved mechanical properties, enhanced stability, altered surface properties, or controlled drug release capabilities [22, 23]. The choice of polymers and grafting techniques depends on the specific application requirements. Polymer-based nanoparticles demonstrate potential in delivering targeted therapeutic treatments for HBV infection, NASH, hepatic fibrosis, HCC, and HVGD [24, 25]. Because of their specific size, shape, stability, chemical makeup, crystal structure, surface area, and other characteristics, nanomaterials have unique optical, mechanical, and electrical properties [26]. TiO2, MoO3, WoO3, CuO, and other metal oxides are less hazardous in nature and have a wide range of uses [27]. TiO2 stands out among them all as a potential contender for various uses because of its special qualities, namely a broad bandgap [28]. Because of the special physical and chemical characteristics of NPs, they have found uses in the biomedical fields. Colloidal NPs are a sort of inorganic substance that has been added to biological fluids to impart optical and magnetic properties [29]. Because of their remarkable pharmacokinetic qualities, NPs are frequently used as drug delivery vehicles, proving their usefulness. Furthermore, there is a lot of interest in how NPs’ catalytic activity can alter the biological microenvironment. Some of these nanoparticles were CuO (copper oxide) and TiO2 (titanium dioxide) nanoparticles, they are widely studied materials with various applications due to their unique properties. CuO Nanoparticles: can be synthesized using various methods, including chemical precipitation [30], thermal decomposition [31], sol-gel synthesis [32], and hydrothermal methods [33]. These methods involve the reduction of copper salts or the thermal decomposition of copper-containing precursors to form CuO nanoparticles. CuO nanoparticles exhibit interesting properties such as high surface area, excellent catalytic activity, semiconductor behavior, and antimicrobial properties. They also possess unique optical, magnetic, and electrical properties, making them suitable for applications in catalysis, energy storage, sensors, electronics, and biomedicine [34,35,36,37]. TiO2 Nanoparticles: can be synthesized using techniques such as sol-gel synthesis [28], hydrothermal methods [38], solvothermal methods [39], and aerosol pyrolysis [40]. These methods involve the hydrolysis and condensation of titanium alkoxides or the thermal decomposition of titanium precursors, resulting in the formation of TiO2 nanoparticles. TiO2 nanoparticles exhibit excellent photocatalytic properties, high refractive index, good optical transparency, and biocompatibility [41]. They are widely utilized in applications such as photocatalysis, solar cells, sensors, environmental remediation, self-cleaning coatings, and biomedical devices [42,43,44]. Both CuO and TiO2 nanoparticles offer unique advantages at the nanoscale due to their increased surface-to-volume ratio, which enhances their reactivity and performance in various applications [45]. It is important to note that the properties and applications of these nanoparticles can be further modified by controlling their size, shape, surface functionalization, and doping [46]. The potential applications of CuO and TiO2 nanoparticles in the medical field are explored, including their antimicrobial properties, drug delivery systems, and diagnostic applications. Additionally, their role in industrial applications, such as catalysis, solar cells, and environmental remediation, is investigated [47]. One approach is to utilize advanced nanomaterials based on natural polymers or industrial polymers as templates or stabilizers for the synthesis of CuNPs. These polymers can help control the size, shape, and stability of the nanoparticles during their formation [48]. Natural Polymers: like chitosan [49, 50], algemate [51], and cellulose [52,53,54]. Industrial Polymers: like Polyvinylpyrrolidone (PVP), Polyethylene glycol (PEG), and Polymethacrylate. The Polymer-Grafted Sodium Alginate Composite Embedded with CuO and TiO2 Nanoparticles faces several bottlenecks and challenges, as well as potential applications. Here are the main ones: Challenges were Synthesis Complexity, Scalability, Nanoparticle Dispersion, and Compatibility and Interactions. On the other hand Possible Applications were Biomedical Materials, Catalysis, Sensors and Electronics, Environmental Applications, and Coatings and FilmsIn this study we synthesized acrylonitrile-co-styrene polymer grafted into sodium alginate and a composite between it and CuO (copper oxide) and TiO2 (titanium dioxide) nanoparticles to study their anticancer and antioxidant activities.
Materials and Methods
Preparation of NaAlg-g-(AN-co-St)
Acrylonitrile (CH2 = CH-CN) (CID 7855) was purchased from (Alpha Chemika, India) and Styrene (C6H5-CH = CH2) (CID 7501) was purchased from (Qualikems, India). Sodium Alginate (C6H7O6Na) (purity 98% and viscosity 31.5 cps; Techno Pharmchem, India). Ammonium persulfate (S2O8(NH4)2) (CID 62648) was purchased from (Piochem, Egypt). DMF (C3H7NO) (CID 6228 ) was purchased from (Sigma, USA).
By free radical polymerization, APS NaAlg-g-(AN-co-St) was synthesized. Sodium alginate solution (0.25 g in 20 mL H2O) was prepared then added AN: St monomers with different ratios ((1:1, 1:3, and 3:1) respectively in 0.5 mol, 1 mol, 2 mol, 2.5 and 3 mol). Heat the reaction mixture to different temperature ranges (85,90,110,120 and 130 °C). The polymerization process began by adding APS with different weights (0.05,0.075,0.1,0.12 and 0.15 mol) dissolved in 10 mL H2O to the reaction mixture. By changing the reaction time (10,15,20,30 and 40 min) the polymer formed then filtered and dried at 40 °C overnight. Where the yield(Y (g)), grafting efficiency (GE %), grafting ratio (GR %), and Conversion (Con %), formed was calculated according to the following Eqs. [55, 56].
Where W0 weight of the monomer, W1 weight of the grafted polymer, and W2 weight of sodium alginate.
Preparation of Nanoparticles
Preparation of CuO Nanoparticles
Using a hydrothermal method [57], CuO nanostructures were fabricated from copper nitrate trihydrate (Cu(NO3)2.3H2O) (CID 9,837,674). Precise control was achieved by dissolving the precursor at 0.1 M concentration and adjusting the pH to 14 with a controlled addition of NaOH. The reaction took place in a customized autoclave at 160 °C for 2 h, followed by thorough washing and drying of the precipitate [58]. Finally, calcination at 500 °C for 4 h yielded the desired CuO nanostructures [59].
Preparation of TiO2 Nanoparticles
5 millilitres of titanium isopropoxide (C12H28O4Ti) (CID 11,026) was gradually added to 8 millilitres of deionized water, leading to the hydrolysis of the alkoxide and the formation of hydrous titanium oxides [60]. The mixture was continuously stirred at 40 °C for 30 min, resulting in the precipitation of titanium dioxide nanoparticles, which appeared as a white solid at the bottom of the beaker [61]. The suspension was then separated by centrifugation, washed several times with deionized water and methanol, and dried in an oven at 80 °C for 12 h [62].
Preparation of Nanocomposite
0.5 gm of grafted polymer was taken and dissolved in 50 mL DMF, stirring until forming a clear solution then add 0.02 gm of CuO or TiO2 stirring until forming a clear solution, and leave it in a petri dish until drying [63, 64].
Characterization
X-ray Diffraction Analysis (XRD)
The analysis was carried out using the 2020964 PANalytical Empyrean, a specialized technique that not only determines the crystal structure of a material (such as graphite or diamond) but also quantifies the relative amounts of each phase present and measures the size of the crystallites.
Scanning Electron Microscopy-Energy Dispersive X-ray Analysis (SEM-EDX)
(SEM) images were acquired using a JEOL (JSM-5200) instrument. To prepare the samples, a small amount of the material was placed onto a carbon tube on a stub, which was then coated with a thin layer of gold. Elemental analysis was performed using an EDX (energy-dispersive X-ray spectroscopy) attachment on a FEG (field emission gun) scanning electron microscope, specifically a Quanta FEG250.
Fourier-Transform Infrared Spectroscopy (FTIR)
Infrared spectra were obtained using the attenuated total reflectance (ATR) technique on a VERTEX 70 Fourier transform infrared (FT-IR) spectrometer. The measurements were carried out at room temperature within the wavenumber range of 4000 to 600 cm− 1.
Thermogravimetric Analysis (TGA)
The thermal analysis was performed using a TGA-50 H thermogravimetric analyzer. The samples were heated from 10 to 600 °C at a rate of 10 °C minutes− 1 in a platinum pan while being purged with nitrogen gas at a flow rate of 25 mL minutes− 1. The analysis was carried out under a nitrogen atmosphere.
Biological Activities
Evaluation of Cytotoxic Effects
Mammalian cell Lines
The American Type Culture Collection (ATCC, Rockville, MD) provided HepG-2 cells (human hepatocellular carcinoma cell line).
Chemicals Used
Sigma (St. Louis, Mo., USA) provided the dimethyl sulfoxide (DMSO), MTT, and trypan blue dye. Lonza (Belgium) provided fetal bovine serum, DMEM, RPMI-1640, HEPES buffer solution, L-glutamine, gentamycin, and 0.25% trypsin-EDTA.
Cell Line Propagation
The cells were cultured in RPMI-1640 media with 10% inactivated fetal calf serum and 50 g/mL gentamycin. The cells were kept at 37 °C in a humidified environment with 5% CO2 and sub-cultured twice or three times per week [65,66,67,68].
The tumour cell lines were suspended in media at a concentration of 5 × 104 cell/well in Corning® 96-well tissue culture plates for antitumor tests, then incubated for 24 h. The compounds were then put into 96-well plates (three replicates) to achieve a total of twelve concentrations for each compound. As a control, six vehicle controls with media or 0.5% DMSO were run for each 96-well plate. The MTT test was used to assess the number of viable cells after 24 h of incubation [69]. In short, the media in the 96 well plate was replaced with 100 µL of fresh culture RPMI 1640 medium without phenol red. Then, 10 µL of a 12 mM MTT stock solution (5 mg of MTT in 1 mL of PBS) was added to each well, including the untreated controls. The plate was incubated at 37 °C and 5% CO2 for 4 h. Afterwards, 85 µL of the media was taken out from each well, and 50 µL of DMSO was added to each well [70]. The contents were mixed thoroughly using a pipette and incubated at 37 °C for 10 min. then, the optical density at 590 nm was measured using a microplate reader (Sunrise, TECAN, Inc, USA) to determine the number of viable cells. The percentage of viability was calculated using the formula[(ODt/ODc)]x100%, where ODt represents the mean optical density of wells treated with the tested sample, and ODc represents the mean optical density of untreated cells [71]. By plotting the relationship between surviving cells and drug concentration, the survival curve of each tumour cell line was obtained after treatment with the specified compound. The 50% inhibitory concentration (IC50), which is the concentration required to cause toxic effects in 50% of intact cells, was estimated by analyzing the dose-response curve using GraphPad Prism software (San Diego, CA. USA) [72].
Evaluation of Antioxidant Activity Using DPPH Scavenging
Antioxidant Assay
The DPPH free radical scavenging assay was performed in triplicate at the Regional Center for Mycology and Biotechnology (RCMB) at Al-Azhar University to determine the antioxidant activity of the extract. The average values from the triplicate measurements were taken into consideration.
DPPH Radical Scavenging Activity
A freshly prepared solution of 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical in methanol with a concentration of 0.004%w/v was made and stored in a dark environment at 10 °C [73]. A methanol solution containing the test compound was also prepared. To initiate the experiment, 40 µL of the methanol solution was added to 3 mL of the DPPH solution [74]. Absorbance measurements were taken immediately using a UV-visible spectrophotometer (Milton Roy, Spectronic 1201) [75]. The decrease in absorbance at 515 nm was continuously monitored, with data recorded at 1-minute intervals until the absorbance reached a stable value, which took 16 min. The absorbance of the DPPH radical without any antioxidant (control), as well as the absorbance of the reference compound ascorbic acid, were also measured. Each measurement was performed in triplicate, and the average values were calculated. The percentage inhibition (PI) of the DPPH radical was calculated according to the formula [76]:
Where AC = Absorbance of the control at t = 0 min and AT = absorbance of the sample + DPPH at t = 16 min.
The 50% inhibitory concentration (IC50), the concentration required to inhibit DPPH radical by 50%, was estimated from graphic plots of the dose-response curve.
Anti-oxidant mechanism
The mechanism of DPPH free radical scavenging involves the following steps [77, 78]:
Initiation: The DPPH radical is initially formed by the reaction of DPPH with a suitable radical initiator, such as a radical-generating compound or exposure to light. The DPPH radical has an unpaired electron, making it highly reactive and capable of abstracting an electron from other molecules.
Antioxidant reaction: When an antioxidant compound, including polymers, is introduced into the DPPH solution, it can interact with the DPPH radical through electron transfer or hydrogen atom transfer mechanisms.
-
a.
Electron transfer: The antioxidant donates an electron to the DPPH radical, which reduces DPPH to its non-radical form. This electron transfer leads to the neutralization of the DPPH radical, resulting in a color change from purple to yellow. The antioxidant is oxidized in the process, but it becomes a stabilized radical itself.
-
b.
Hydrogen atom transfer: Alternatively, the antioxidant can donate a hydrogen atom to the DPPH radical, resulting in the formation of the non-radical form of DPPH and the antioxidant radical. Again, this hydrogen atom transfer leads to the neutralization of the DPPH radical and a color change in the solution.
Measurement: The reduction of DPPH is typically monitored spectrophotometrically by measuring the decrease in absorbance at a specific wavelength, usually around 517 nm. The extent of the color change is proportional to the antioxidant activity of the compound being tested. A stronger antioxidant will cause a more significant reduction in absorbance, indicating higher free radical scavenging activity.
Results and Discussion
Effect of the Different Reaction Parameters on the Grafting Process
By investigating various factors such as initiator concentration, monomer concentration, reaction temperature, and reaction time, the optimum parameters for grafting were determined and are outlined below. The effectiveness of the grafting process was assessed based on the yield of the resulting graft. Regarding the initiator concentration, the range examined was 0.05 to 0.15 mol/L. The highest graft yield observed was 5.25 g at an initiator concentration of 0.12 mol/L. The study also investigated different monomer ratios, with a range of 0.5 to 3-mol ratios, considering varying ratios of acrylonitrile (AN) to styrene (St) (1:1, 1:3, and 3:1). It was found that the optimal molar ratio was 1 AN to 3 St, resulting in a graft yield of 13.4 g. The impact of reaction time (ranging from 10 to 40 min) was examined. The highest graft yield, 13.4 g, was achieved at a reaction time of 20 min. Furthermore, the effect of temperature (ranging from 85 to 130 °C) on the grafting process was investigated. The highest graft yield of 13.4 g was obtained at a temperature of 110 °C. These data are summarized in Table 1.
Formation of Nanocomposite
Figure 1. represents the mechanism of formation of the final designed nanocomposite (NaAlg-g-(AN-co-St) composites containing Cu, Ti). We can see that a high OH groups ratio, it is responsible for reducing many metal ions to nanoparticles. This hypothesis has been confirmed by several published reports on reducing various metal ions, such as Cu2+, Se2+, Ti4+ Fe3+,and Zn2+, using plant extracts [64].
X-Ray Diffraction Analysis (XRD) for the Prepared Samples
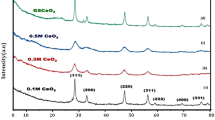
Figure 2. represents the XRD graph for the prepared composites. We can notice first for the polymer that the sample is amorphous as reported in the literature [79]. The Na-alginate sample showed the same result compared to the reported data [80]. To evaluate and confirm the formation of the composites we can notice for the CuO sample the three diffraction peaks appeared at angles 33.72°, 35.51°, and 38.78º corresponding to (110), (-111), and (111) for the tenorite phase of CuO following the card no. 00-005-0061 with monoclinic crystallography [81]. Related to the composite with TiO2 we can that impeding the TiO2 nanoparticles improves the crystallinity of the composite and three diffraction peaks appeared at angles 23.80°, 35.78°, and 48.41º corresponding to (101), (103), and (200) for the anatase phase of TiO2 following the card no. 84-1285 [82]. Finally, in the composite sample, we noticed an improvement in the crystallinity of the composite that will play a significant role in the application part most of the peaks overlapped but still the peaks (111), and (200) related to both CuO and TiO2 respectively can be noticed. The average crystallite size for the prepared CuO and TiO2 nanoparticles can be calculated from the following Scherrer’s equation [83]
Where, D: crystal size, λ: X- ray wave length, β: the broadening of the diffraction peak and Ɵ is the diffraction angle.
From the results the average crystallite size for the CuO, and TiO2 nanoparticles are 15, 18 nm respectively.
Scanning Electron Microscopy-Energy Dispersive X-ray Analysis (SEM-EDX)
As we can see in Fig. 3 (a,b) the average particle size for the prepared CuO and TiO2 nanoparticles is 20–25 nm. Figure 3.(c-d) The EDX results for the prepared composites confirm the successive impeding CuO, and TiO2 in the composite. Figure 3 (a,b). represents the SEM results for the prepared composite, we can notice by adding CuO nanoparticles to the composite (Fig. 3c), and TiO2 nanoparticles to the composite (Fig. 3d) formed nanopores that improve the composite for the application part. Related to Fig. 3e which represents composites with both CuO and TiO2 nanoparticles, we can notice that the crystallinity is improved, and the particle size is reduced which will play a significant role in the application part.
Fourier-Transform Infrared Spectroscopy (FTIR)
Changes in the structures of Na-alginate and polymer were confirmed by FTIR, Fig. (4) shows the most characteristic peak of Na-alginate which observed a peak at 3450 cm− 1 which indicates that the presence of OH (hydroxyl group), absorption band around 1740, 1625 and 1340 cm− 1 are attributed to symmetric and asymmetric carboxylate anion [84], the evidence for the formation of NaAlg-g-(AN-co-St) was confirmed by the weak intensity of OH (Hydroxyl group) and appear new peaks for (AN-co-St) at 2230 cm− 1 for CN, 1498 cm− 1 for C = C phenyl ring and 1100 cm− 1 for C-H bending for phenyl group [85], on the other hand the formation of composites were supported by appear a new peaks at 650 cm− 1 for CuO [86], 730 and 750 cm− 1 for Ti-O-Ti [87] and the presence of peaks for CuO and TiO2 at the last graph indicated that formation the composite between grafted polymer and both of them.
Thermogravimetric Analyses
Figure 0.5. presents the results of the thermogravimetric analysis (TGA) conducted on sodium alginate. The TGA curve revealed a two-step weight loss pattern. The initial weight loss, accounting for approximately 16% of the total weight, occurred within the temperature range of 30–125 °C. This weight loss was attributed to the evaporation of moisture, which has been previously documented by other researchers [88]. In the case of sodium alginate, the second weight loss, accounting for approximately 40% of the total weight, occurred within the temperature range of 200–250 °C. This weight loss was attributed to a complex process that resulted in the degradation of the sample. During this process, water, methane (CH4), and carbon dioxide (CO2) were formed and released. The temperature at which 50% of the material’s weight was lost was observed to be 260 °C.
On the other hand, for NaAlg-g-(AN-co-St) (shown in Fig. 5), the first step of weight loss, approximately 15%, began at 200–250 °C. This weight loss was also due to a complex process involving the degradation of carboxylate groups (COO-) resulting in the release of CO2, as well as the formation of methane and water. The second step of weight loss, accounting for 42% of the total weight, occurred at 300–500 °C. This weight loss can be attributed to the degradation of the grafted polyacrylonitrile chains and ultimately the formation of char from inorganic constituents [89]. The temperature at which the copolymer sample experienced a 50% weight loss was measured to be 380 °C, indicating that the graft copolymer exhibits enhanced thermal stability compared to sodium alginate alone.
In the case of grafted polymer-metal oxide composites (shown in Fig. 5), for the copper oxide composite, the initial weight loss of approximately 10% occurred at 190 °C, followed by a second weight loss of around 15% at 300 °C. The final weight loss of about 75% was observed at 450 °C.
For the titanium oxide composite, two weight losses were observed, with the first loss accounting for roughly 30% occurring at 300 °C, and the second loss of about 90% happening at 450 °C.
In the case of the copper-titanium oxide composite, there were two weight losses as well, with the first loss of approximately 15% occurring at 290 °C, followed by a second loss of around 50% at 450 °C.
These results indicate that the incorporation of metal oxides increased the thermal stability of the prepared polymers in all cases.
Biological Activity
Cytotoxicity
The half-maximal inhibitory concentration (IC50) is a commonly used and informative metric to evaluate the effectiveness of a drug. It quantifies the amount of drug required to inhibit a biological process by 50%, thereby providing a measure of the potency of an antagonist drug in pharmacological research [90, 91]. The in vitro cytotoxicity of the synthesized compounds against the human hepatocellular cancer cell line (HepG-2) was assessed using the MTT test. The full form of MTT was given in Fig. (6). The dose-response curves can be found in Fig. 6a-e. Overall, the results demonstrated that increasing the concentration of the compounds led to a significant decrease in cell viability for the HepG-2 cell line.
In particular, the parent compound alginic acid (Alg) exhibited the lowest activity against HepG-2 cells, as its highest concentration (500 µg/mL) resulted in the highest percentage of cell viability (Table 2; Fig. 7a). The IC50 values further confirmed that Alg was the least active compound against HepG-2 cells (IC50 > 500 µg/mL). On the other hand, the polymerized form of SA-(AN-co-St) showed higher activity against HepG-2 cells, with an IC50 value of 445.37 ± 17.23 µg/mL. For the NaAlg-g-(AN-co-St) composites containing Cu, Ti, and Cu-Ti oxides, it can be observed that NaAlg-g-(AN-co-St)-(Ti-Cu oxides) exhibited greater activity against HepG-2 cells compared to NaAlg-g-(AN-co-St) and NaAlg-g-(AN-co-St) composites with Cu and Ti oxides (Fig. 7b-c; Table 2), with an IC50 value of 96.11 ± 2.95 µg/mL. The inclusion of metal oxides (Cu, Ti, and Cu-Ti) enhanced the cytotoxic activity of the prepared polymers [92, 93].
HePG-2 (human Hepatocellular carcinoma) of (a) sodium alginate (NaAlg), (b) sodium alginate polymerized acrylonitrile-Styrene NaAlg-g-(AN-co-St), (c) NaAlg-g-(AN-co-St)composite with copper oxide (d) NaAlg-g-(AN-co-St)composite with Titanium oxide (e) NaAlg-g-(AN-co-St)composite with (cupper-Titanium oxide)
The toxicity process against HepG-2 cells involves a series of mechanisms that ultimately lead to cell death
Cellular Uptake: The fabricated polymer and its metal oxide nanocomposites are taken up by the HepG-2 cells. The nanocomposites may enter the cells through various mechanisms, including endocytosis or direct penetration of the cell membrane.
Reactive Oxygen Species (ROS) Generation: Once inside the cells, metal oxide nanoparticles, such as copper oxide (CuO) and titanium oxide (TiO2), can interact with cellular components and initiate oxidative stress. These metal oxides have the ability to generate reactive oxygen species (ROS), including superoxide anions (O2•−) and hydroxyl radicals (•OH). The excessive production of ROS can disrupt cellular redox balance and lead to oxidative damage.
Oxidative Stress: The accumulation of ROS results in oxidative stress, which causes damage to cellular structures, including lipids, proteins, and DNA. ROS can directly oxidize and modify biomolecules, leading to cellular dysfunction and impairment of essential cellular processes.
Mitochondrial Dysfunction: ROS can target mitochondria, which are the energy-producing organelles within the cells. Oxidative damage to mitochondrial components, such as the mitochondrial membrane, can disrupt the electron transport chain and ATP production. This leads to mitochondrial dysfunction, a critical event in the toxicity process, as it further exacerbates oxidative stress and impairs cellular energy metabolism.
Activation of Signaling Pathways: The oxidative stress and mitochondrial dysfunction triggered by the nanocomposites activate various signaling pathways within the cells. These pathways may include the MAPK (mitogen-activated protein kinase) pathway, the PI3K/Akt (phosphoinositide 3-kinase/protein kinase B) pathway, and the p53 pathway. Activation of these pathways can modulate cell survival, proliferation, and apoptosis.
DNA Damage and Cell Cycle Arrest: The oxidative stress and activation of signaling pathways can cause DNA damage in HepG-2 cells. DNA damage can lead to cell cycle arrest, preventing cells from progressing through the cell cycle and initiating DNA repair mechanisms. If the damage is severe and irreparable, it can trigger programmed cell death or apoptosis.
Induction of Apoptosis: The toxicity process often culminates in the induction of apoptosis, a programmed cell death mechanism. Apoptosis involves a series of biochemical events, including activation of caspases (proteases), DNA fragmentation, and cellular shrinkage. These events ultimately lead to the dismantling and removal of the damaged cells.
EtOH exposure has been shown to induce cytotoxic effects on HepG-2 cells. In vitro studies have demonstrated that high concentrations of EtOH can lead to cell death through various mechanisms, including oxidative stress, mitochondrial dysfunction, and apoptosis. The cytotoxic effects of EtOH are dose-dependent, with higher concentrations generally causing more pronounced damage to the cells [94].
Antioxidant Activity
The DPPH assay is a commonly used and straightforward colourimetric technique for determining antioxidant activity [95, 96]. The DPPH assay is widely employed to assess the radical scavenging activity of natural compounds, particularly for evaluating the antioxidant properties of phenolic compounds [97]. In this study, the DPPH radical scavenging assay was utilized to evaluate the antioxidant activity of NaAlg-g-(AN-co-St) and its metal oxide nanocomposites (Cu, Ti, and Cu-Ti). The results presented in Fig. 8 (a-e) and Table 3 indicate that the unmodified alginate (SA) exhibited low antioxidant activity with an IC50 value of 462.03 ± 14.03 µg/mL (Fig. 8a). Following grafting with the AN-co-St copolymers, an increase in antioxidant activity was observed with an IC50 value of 237.39 ± 3.87 µg/mL (Fig. 8b). This enhancement can be attributed to the introduction of new copolymers. Upon incorporating metal oxides, we observed high antioxidant activity with IC50 values of 228.25 ± 4.09 µg/mL, 188.60 ± 4.31 µg/mL, and 146.10 ± 5.72 µg/mL for copper oxide, titanium oxide, and copper-titanium oxides, respectively (Fig. 8c-e), which can be attributed to the high antioxidant activity of the incorporated metal oxides [92, 93].
Evaluation of Antioxidant Activity using DPPH scavenging (a) sodium alginate (NaAlg), (b) sodium alginate polymerized acrylonitrile-Styrene NaAlg-g-(AN-co-St), (c) NaAlg-g-(AN-co-St)composite with copper oxide (d) NaAlg-g-(AN-co-St)composite with Titanium oxide (e) NaAlg-g-(AN-co-St)composite with (cupper-Titanium oxide)
Conclusion
In this study, the optimal molar ratio of 1 AN to 3 St was determined, resulting in a graft yield of 13.4 g with a reaction time of 20 min and a temperature of 110 °C. These findings, summarized in Table 1, provide a comprehensive overview of the optimal parameters for grafting. Researchers can now apply these specific conditions to achieve the highest possible graft yield in similar grafting processes.
The results of this study demonstrate the successful preparation of a composite material consisting of sodium alginate-grafted-(acrylonitrile-co-styrene) (NaAlg-g-(AN-co-St)) and metal oxides (Cu, Ti, and Cu-Ti). The composite exhibited significantly higher cytotoxic activity compared to the composites containing individual metal oxides. Specifically, the composite displayed an IC50 value of 96.11 ± 2.95 µg/mL, indicating its increased potency against HepG-2 cells, a cell line commonly used to study liver cancer.
The improved cytotoxic activity of the composite can be attributed to the inclusion of metal oxides. Metal oxides, such as copper oxide (CuO) and titanium oxide (TiO2), have been reported to possess intrinsic cytotoxic properties [98]. These metal oxides can generate reactive oxygen species (ROS) and induce oxidative stress in cells, leading to cell death. The synergistic effect of Cu and Ti oxides in the composite may further enhance the cytotoxic activity by promoting ROS generation and oxidative damage [99].
The grafting of acrylonitrile (AN) and styrene (St) onto sodium alginate (NaAlg) provides additional benefits to the composite [100]. AN and St are known to possess bioactive properties and can potentially enhance the cytotoxic effects of the composite. Moreover, the presence of NaAlg in the composite may facilitate controlled drug release, as NaAlg has been widely investigated as a drug delivery system due to its biocompatibility and biodegradability [101].
The observed cytotoxic activity of the composite against HepG-2 cells suggests its potential for pharmacological applications targeting liver cancer. HepG-2 cells are often used as a model for studying liver cancer, and compounds with cytotoxic activity against these cells hold promise for the development of novel anticancer therapies [102]. The synthesized composite could be further explored as a potential candidate for liver cancer treatment, either as a standalone therapeutic agent or in combination with other treatment modalities.
The effectiveness of the fabricated polymer (NaAlg-g-(AN-co-St)) and its metal oxide nanocomposites (Cu, Ti, and Cu-Ti) against HepG-2 cells can be attributed to several factors:
Cytotoxicity of metal oxides: Copper oxide (CuO) and titanium oxide (TiO2) have been reported to exhibit cytotoxic effects against various cancer cell lines, including HepG-2 cells. These metal oxides can induce oxidative stress, generate reactive oxygen species (ROS), and disrupt cellular processes, ultimately leading to cell death. The incorporation of these metal oxides in the nanocomposites contributes to their cytotoxic activity against HepG-2 cells [103].
Synergistic effects: The combination of copper oxide and titanium oxide in the Cu-Ti nanocomposite may result in synergistic effects, where the combined action of the two metal oxides enhances the overall cytotoxicity. Synergy between different compounds can lead to increased efficacy and potency, potentially resulting in a stronger cytotoxic response against targeted cells [104].
Enhanced cellular uptake: The presence of metal oxide nanoparticles in the nanocomposite can facilitate enhanced cellular uptake by HepG-2 cells. Nanoparticles have been found to exhibit increased cellular internalization compared to bulk materials, allowing for more efficient delivery of the active compounds. The enhanced cellular uptake may lead to higher concentrations of the cytotoxic agents within the cells, thereby increasing their effectiveness against HepG-2 cells [105].
Polymer matrix effects: The polymer matrix, NaAlg-g-(AN-co-St), may also contribute to the cytotoxic activity against HepG-2 cells. The grafting of acrylonitrile (AN) and styrene (St) onto sodium alginate (NaAlg) can introduce bioactive properties and enhance the overall cytotoxic effects. Additionally, the polymer matrix may provide stability and controlled release of the active compounds, ensuring their prolonged exposure to the cancer cells [106].
The evaluation of the antioxidant activity of NaAlg-g-(AN-co-St) and its metal oxide nanocomposites (Cu, Ti, and Cu-Ti) using the DPPH assay provides valuable insights into their potential as antioxidants. The DPPH assay is a widely used method to assess the ability of compounds to scavenge free radicals and is indicative of their antioxidant capacity.
The results of this study demonstrate the effectiveness of the synthesized compounds in scavenging DPPH radicals, indicating their antioxidant activity. The incorporation of metal oxides, including copper oxide (CuO), titanium oxide (TiO2), and copper-titanium oxides, significantly enhanced the antioxidant activity of the nanocomposites [107]. The IC50 values obtained for the nanocomposites (228.25 ± 4.09 µg/mL for CuO, 188.60 ± 4.31 µg/mL for TiO2, and 146.10 ± 5.72 µg/mL for Cu-Ti oxides) indicate their ability to inhibit DPPH radicals effectively [108]. The observed increase in antioxidant activity can be attributed to the intrinsic antioxidant properties of the incorporated metal oxides. Copper oxide and titanium oxide are known to possess antioxidant activity due to their ability to scavenge free radicals and inhibit oxidative stress [109]. These metal oxides can donate electrons or hydrogen atoms to neutralize free radicals, thereby preventing oxidative damage to cells and biomolecules. The synergistic effect of copper and titanium oxides in the Cu-Ti nanocomposite may further enhance the antioxidant activity, potentially through cooperative interactions between the two metal oxides.
The antioxidant activity demonstrated by the nanocomposites suggests their potential applications in various fields, including food preservation, cosmetics, and pharmaceuticals. Antioxidants play a crucial role in preventing or reducing oxidative stress-induced damage and are sought after for their potential health benefits. The synthesized nanocomposites, with their enhanced antioxidant activity, could be explored as natural and sustainable alternatives to synthetic antioxidants in these applications.
However, it should be noted that the DPPH assay provides an initial assessment of antioxidant activity in vitro and does not fully reflect the complex interactions and mechanisms that occur in vivo. Further studies, including in vitro cellular models and in vivo animal studies, would be necessary to evaluate the bioavailability, bioactivity, and potential side effects of these nanocomposites [110].
Overall, these findings indicate that the synthesized compounds, particularly the metal oxide nanocomposites, possess significant antioxidant activity as demonstrated by their ability to scavenge DPPH radicals. This suggests their potential application as effective antioxidants in various fields, including food science and pharmaceuticals, where antioxidant properties are highly desirable.
Data Availability
Sharing data is not possible for this topic because no files were made or analyzed.
References
Yang Q et al (2023) Human proteome microarray identifies autoantibodies to tumor-associated antigens as serological biomarkers for the diagnosis of hepatocellular carcinoma. Mol Oncol 17(5):887–900
Gutiérrez-Cuevas J et al (2022) Epidemiologic, genetic, pathogenic, metabolic, epigenetic aspects involved in Nash-hcc: current therapeutic strategies. Cancers 15(1):23
Liu Y, Liu L (2022) Changes in the epidemiology of hepatocellular carcinoma in Asia. Cancers 14(18):4473
Rumgay H et al (2022) Global, regional and national burden of primary liver cancer by subtype. Eur J Cancer 161:108–118
Huang DQ et al (2022) Changing global epidemiology of liver cancer from 2010 to 2019: NASH is the fastest growing cause of liver cancer. Cell Metabol 34(7):969–977e2
Ghamari SH et al (2022) Trends in Global, Regional, and National Burden and Quality of Care Index for Liver Cancer by cause from global burden of Disease 1990-2019. Hepatol Commun 6(7):1764–1775
Chartampilas E et al (2022) Current imaging diagnosis of hepatocellular carcinoma. Cancers 14(16):3997
Huang L et al (2023) Rapid, label-free histopathological diagnosis of liver cancer based on Raman spectroscopy and deep learning. Nat Commun 14(1):48
Renzulli M et al (2022) The feasibility of liver biopsy for undefined nodules in patients under Surveillance for Hepatocellular Carcinoma: is Biopsy really a useful Tool? J Clin Med 11(15):4399
Ahmad J et al (2022) Value of liver biopsy in the diagnosis of drug-induced liver injury. J Hepatol 76(5):1070–1078
Brar G et al (2022) Redefining intermediate-stage HCC treatment in the era of immune therapies. JCO Oncol Pract 18(1):35–41
Semmler G et al (2022) HCC risk stratification after cure of hepatitis C in patients with compensated advanced chronic liver disease. J Hepatol 76(4):812–821
Xie J et al (2022) An immune subtype-related prognostic signature of hepatocellular carcinoma based on single-cell sequencing analysis. Aging 14(7):3276
Muthulakshmi L et al (2023) Sodium Alginate Grafted Biopolymers as Corrosion Inhibitors. Safety, Sustainability, and Efficiency
Hegde V et al (2022) Alginate based polymeric systems for drug delivery, antibacterial/microbial, and wound dressing applications. Mater Today Commun, : p. 104813
Yan Y et al (2022) Synthesis and characterization of protocatechuic acid grafted carboxymethyl chitosan with oxidized sodium alginate hydrogel through the Schiff’s base reaction. Int J Biol Macromol 222:2581–2593
Wu S et al (2022) Efficient removal of cr (VI) by triethylenetetramine modified sodium alginate/carbonized chitosan composite via adsorption and photocatalytic reduction. J Mol Liq 366:120160
Wei Q et al (2023) Modification, 3D printing process and application of sodium alginate based hydrogels in soft tissue engineering: a review. Int J Biol Macromol, : p. 123450
Masod MB, El-Fiqi A, Ebiad MA (2023) Encapsulation of activated carbon into calcium alginate microspheres toward granular-activated carbon adsorbents for elemental mercury capture from natural gas. Environmental Science and Pollution Research, pp 1–18
Rimmele M, Glöcklhofer F, Heeney M (2022) Post-polymerisation Approaches Rapid Modif Conjugated Polym Prop Mater Horizons
Keyes A et al (2022) Tandem living insertion and controlled radical polymerization for polyolefin–polyvinyl block copolymers. Angew Chem 134(10):e202112742
Naeem A et al (2023) Study of Hydroxypropyl β-Cyclodextrin and puerarin inclusion complexes encapsulated in Sodium Alginate-grafted 2-Acrylamido-2-Methyl-1-Propane sulfonic acid hydrogels for oral controlled drug delivery. Gels 9(3):246
Bhatt P et al (2023) Plasma modification techniques for natural polymer-based drug delivery systems Pharmaceutics, 15(8): p. 2066
Luo F et al (2022) Polymeric nanomedicines for the treatment of hepatic diseases. J Nanobiotechnol 20(1):1–28
Vaughan HJ et al (2022) Polymeric nanoparticles for dual-targeted theranostic gene delivery to hepatocellular carcinoma. Sci Adv 8(29):eabo6406
Ahmad MM et al (2022) Green synthesis of mn + cu bimetallic nanoparticles using Vinca rosea extract and their antioxidant, antibacterial, and catalytic activities. Crystals 12(1):72
Murthy HA et al (2021) Enhanced multifunctionality of CuO nanoparticles synthesized using aqueous leaf extract of Vernonia amygdalina plant. Results Chem 3:100141
Ahmad MM et al (2021) Investigation of TiO2 nanoparticles synthesized by sol-gel method for effectual photodegradation, oxidation and reduction reaction. Crystals 11(12):1456
Zeebaree SYS, Zeebaree AYS, Zebari OIH (2020) Diagnosis of the multiple effect of selenium nanoparticles decorated by Asteriscus graveolens components in inhibiting HepG2 cell proliferation. Sustainable Chem Pharm 15:100210
Che J et al (2022) An efficient process for recovering copper as CuO nanoparticles from acidic waste etchant via chemical precipitation and thermal decomposition: turning waste into value-added product. J Clean Prod 369:133404
Benhammada A, Trache D (2022) Green synthesis of CuO nanoparticles using Malva sylvestris leaf extract with different copper precursors and their effect on nitrocellulose thermal behavior. J Therm Anal Calorim 147:1–16
Patel M et al (2022) Synthesis of ZnO and CuO nanoparticles via Sol gel method and its characterization by using various technique. Discover Mater 2(1):1
Chandrasekar M et al (2022) Synthesis and characterization studies of pure and Ni doped CuO nanoparticles by hydrothermal method. J King Saud University-Science 34(3):101831
Iqbal T et al (2022) Green synthesis of novel lanthanum doped copper oxide nanoparticles for photocatalytic application: correlation between experiment and COMSOL simulation. Ceram Int 48(10):13420–13430
Ning Y et al (2022) Exploring the spindle-shaped copper oxide nanoparticles as cost‐effective Catalyst. ChemistrySelect 7(19):e202200626
Maliki M et al (2022) Copper nanoparticles and their oxides: optical, anticancer and antibacterial properties. Int Nano Lett 12(4):379–398
Sarfraz S et al (2020) Copper oxide nanoparticles: reactive oxygen Species Generation and Biomedical Applications. Int J Comput Theor Chem 8:40–46
Le T-LT et al (2021) Controlled growth of TiO2 nanoparticles on graphene by hydrothermal method for visible-light photocatalysis. J Science: Adv Mater Devices 6(4):516–527
Ramakrishnan VM et al (2020) Performance of TiO2 nanoparticles synthesized by microwave and solvothermal methods as photoanode in dye-sensitized solar cells (DSSC). Int J Hydrog Energy 45(51):27036–27046
Ismail MA et al (2021) Synthesis and characterization of iron-doped TiO2 nanoparticles using ferrocene from flame spray pyrolysis. Catalysts 11(4):438
Mohamed HS et al (2021) Nano metal oxide impregnated Chitosan-4-nitroacetophenone for industrial dye removal. Int J Environ Anal Chem 101(13):1850–1877
Fathi N, Almasi H, Pirouzifard MK (2019) Sesame protein isolate based bionanocomposite films incorporated with TiO2 nanoparticles: study on morphological, physical and photocatalytic properties. Polym Test 77:105919
Tudu BK, Sinhamahapatra A, Kumar A (2020) Surface modification of cotton fabric using TiO2 nanoparticles for self-cleaning, oil–water separation, antistain, anti-water absorption, and antibacterial properties. ACS Omega 5(14):7850–7860
Samhitha SS et al (2022) Green synthesized TiO2 nanoparticles for anticancer applications: Mini review Materials Today: Proceedings, 54: p. 765–770
Soliman N et al (2021) Experimentally and theoretically approaches for disperse red 60 dye adsorption on novel quaternary nanocomposites. Sci Rep 11(1):10000
Zebari OIH et al (2019) Antibacterial activity of copper nanoparticles fabricate via malva sylvesteris leaf extract. Kurdistan J Appl Res, : p. 146–156
Zeebaree AYS et al (2022) Sustainable engineering of plant-synthesized TiO2 nanocatalysts: diagnosis, properties and their photocatalytic performance in removing of methylene blue dye from effluent. A review. Curr Res Green Sustainable Chem 5:100312
Yasin SA et al (2021) The efficient removal of methylene blue dye using CuO/PET nanocomposite in aqueous solutions. Catalysts 11(2):241
Zeebaree AYS et al (2023) Current contributions of novel nanoparticles-based colorimetric sensors for detection of SCN ions in different aqueous models. A review. Arab J Chem, : p. 105297
Raouf OH et al (2020) Synthesis, characterization and biological activity of Schiff bases based on chitosan and acetophenone derivatives. AJCA 3:274–282
Zeebaree SYS, Zeebaree AYS (2019) Synthesis of copper nanoparticles as oxidising catalysts for multi-component reactions for synthesis of 1, 3, 4-thiadiazole derivatives at ambient temperature. Sustainable Chem Pharm 13:100155
Zeebaree SYS et al (2022) Novel natural exudate as a stabilizing agent for fabrication of copper nanoparticles as a colourimetric sensor to detect trace pollutant. Surf Interfaces 32:102131
Zeebaree SYS et al (2021) Sustainable fabrication, optical properties and rapid performance of bio-engineered copper nanoparticles in removal of toxic methylene blue dye in an aqueous medium. Curr Res Green Sustainable Chem 4:100103
Mohamed HS et al (2022) Adsorption of mn + 7 ions on chitosan/cellulose composite: experimentally and theoretically approaches. J Dispers Sci Technol 43(10):1525–1542
Craciun G et al (2017) Obtaining a new type of polyelectrolyte based on acrylamide and hydrolyzed collagen by electron beam irradiation. Polym Bull 74:1299–1326
Craciun G, Manaila E, Ighigeanu D (2019) New type of sodium alginate-g-acrylamide polyelectrolyte obtained by electron beam irradiation: characterization and study of flocculation efficacy and heavy metal removal capacity. Polymers 11(2):234
Jiang T et al (2014) Controllable fabrication of CuO nanostructure by hydrothermal method and its properties. Appl Surf Sci 311:602–608
Dar MA et al (2010) Synthesis, characterization, and electrochemical properties of self-assembled leaf-like CuO nanostructures. J Solid State Electrochem 14:1719–1726
Dar M et al (2008) Structural and magnetic properties of CuO nanoneedles synthesized by hydrothermal method. Appl Surf Sci 254(22):7477–7481
Nakayama N, Hayashi T (2008) Preparation of TiO2 nanoparticles surface-modified by both carboxylic acid and amine: dispersibility and stabilization in organic solvents. Colloids Surf a 317(1–3):543–550
Ali HM et al (2018) Preparation techniques of TiO2 nanofluids and challenges: a review. Appl Sci 8(4):587
Politova-Brinkova NI et al (2020) Preparation of TiO2 Nanoparticle aggregates and capsules by the ‘Two-Emulsion method’. Colloids Interfaces 4(4):57
Ishida H, Campbell S, Blackwell J (2000) General approach to nanocomposite preparation. Chem Mater 12(5):1260–1267
Sharaf Zeebaree SY et al (2022) Rapid detection of mercury ions using sustainable natural gum-based silver nanoparticles. Catalysts 12(11):1464
Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65(1–2):55–63
Gomha S et al (2015) Synthesis and anticancer activities of thiazoles, 1, 3-thiazines, and thiazolidine using chitosan-grafted-poly (vinylpyridine) as basic catalyst. Heterocycles 91(6):1227–1243
Mokhtar FY et al (2023) Bioactive secondary metabolites from aspergillus fumigatus ON428521 isolated from Wadi El Rayan, El Fayum Governorate. Egypt J Bot 63(1):233–250
Hamza RA et al (2022) Cytotoxic activity of some Egyptian plants against hepatic human cancer cell line, in-vivo anticancer activity and bio-guided isolation of active extracts. Egypt J Chem 65(132):1465–1476
Kamiloglu S et al (2020) Guidelines for cell viability assays. Food Front 1(3):332–349
Buranaamnuay K (2021) The MTT assay application to measure the viability of spermatozoa: a variety of the assay protocols. Open Veterinary J 11(2):251–269
Sayed MA et al (2022) Attractive study of the antimicrobial, antiviral, and cytotoxic activity of novel synthesized silver chromite nanocomposites. BMC Chem 16(1):39
Le Berre M et al (2022) Calculating half maximal inhibitory concentration (IC 50) values from glycomics microarray data using graphpad prism. Glycan Microarrays: Methods Protocols, : p. 89–111
Gulcin İ, Alwasel SH (2023) DPPH radical scavenging assay. Processes 11(8):2248
Marinova G, Batchvarov V (2011) Evaluation of the methods for determination of the free radical scavenging activity by DPPH. Bulgarian J Agricultural Sci 17(1):11–24
Locatelli M et al (2009) Study of the DPPH-scavenging activity: development of a free software for the correct interpretation of data. Food Chem 114(3):889–897
Jiménez-Escrig A et al (2000) Evaluation of free radical scavenging of dietary carotenoids by the stable radical 2, 2‐diphenyl‐1‐picrylhydrazyl. J Sci Food Agric 80(11):1686–1690
Seguchi T et al (2012) Mechanism of antioxidant interaction on polymer oxidation by thermal and radiation ageing. Radiat Phys Chem 81(11):1747–1751
Al-Malaika S, Scott G (1983) Mechanisms of antioxidant action. Effect of the polymer medium on the antioxidant efficiency of the dithiolates. Eur Polymer J 19(3):241–246
Peng Z, Ye L, Ade H (2022) Understanding, quantifying, and controlling the molecular ordering of semiconducting polymers: from novices to experts and amorphous to perfect crystals. Mater Horiz 9(2):577–606
Permatasari AAAP et al (2022) Extraction and characterization of sodium alginate from three brown algae collected from Sanur Coastal Waters, Bali as Biopolymer agent. Biodiversitas J Biol Divers, 23(3)
Feng L et al (2019) In situ XRD observation of CuO anode phase conversion in lithium-ion batteries. J Mater Sci 54(2):1520–1528
Bokuniaeva AO, Vorokh AS Estimation of particle size using the Debye equation and the Scherrer formula for polyphasic TiO2 powder. IOP Publishing
Miranda M, Sasaki J (2018) The limit of application of the Scherrer equation. Acta Crystallogr Sect A: Found Adv 74(1):54–65
Badawi NM et al (2023) Highly conductive and reusable cellulose hydrogels for Supercapacitor Applications. Micromachines 14(7):1461
Broglia MF et al (2015) Rapid fabrication of periodic patterns on poly (styrene-co-acrylonitrile) surfaces using direct laser interference patterning International Journal of Polymer Science, 2015
Ahmad S, Saeed A (2019) Synthesis of metal/silica/titania composites for the photocatalytic removal of methylene blue dye Journal of Chemistry, 2019
Zhang W et al (2020) Construction of NiO/H 2 Ti 3 O 7 nanotube composite and its photocatalytic conversion feature for ethyl mercaptan. Appl Phys A 126:1–10
Srinivasan SS et al (2010) Reversible hydrogen storage in electrospun polyaniline fibers. Int J Hydrog Energy 35(1):225–230
Salisu A et al (2016) Alginate graft polyacrylonitrile beads for the removal of lead from aqueous solutions. Polym Bull 73:519–537
Aykul S, Martinez-Hackert EJAb (2016) Determination of half-maximal inhibitory concentration using biosensor-based protein interaction analysis 508: p. 97–103
Aykul S, Martinez-Hackert E (2016) Determination of half-maximal inhibitory concentration using biosensor-based protein interaction analysis. Anal Biochem 508:97–103
Kaningini AG et al (2023) Antimicrobial, antioxidant, and cytotoxic properties of biosynthesized copper oxide nanoparticles (CuO-NPs) using Athrixia phylicoides DC. Heliyon, 9(4)
Mbenga Y et al (2023) Green synthesis, antioxidant and anticancer activities of TiO2 nanoparticles using aqueous extract of Tulbhagia Violacea. Results in Chemistry, p 101007
Gutiérrez-Ruiz MC et al (1999) Cytokines, growth factors, and oxidative stress in HepG2 cells treated with ethanol, acetaldehyde, and LPS. Toxicology 134(2–3):197–207
Sharma OP, Bhat TK (2009) DPPH antioxidant assay revisited. Food Chem 113(4):1202–1205
Zamani M, Delfani AM, Jabbari M (2018) Scavenging performance and antioxidant activity of γ-alumina nanoparticles towards DPPH free radical: Spectroscopic and DFT-D studies. Spectrochim Acta Part A Mol Biomol Spectrosc 201:288–299
Srivastava A, Rao LJM, Shivanandappa T (2012) A novel cytoprotective antioxidant: 4-Hydroxyisophthalic acid. Food Chem 132(4):1959–1965
Stankic S et al (2016) Pure and multi metal oxide nanoparticles: synthesis, antibacterial and cytotoxic properties. J Nanobiotechnol 14(1):1–20
Vimbela GV et al (2017) Antibacterial properties and toxicity from metallic nanomaterials. Int J Nanomed, : p. 3941–3965
Chen M et al (2021) Kaolin-enhanced superabsorbent composites: synthesis, characterization and swelling behaviors. Polymers 13(8):1204
Pawar SN, Edgar KJ (2012) Alginate derivatization: a review of chemistry, properties and applications. Biomaterials 33(11):3279–3305
Bennani FE et al (2023) 2D-QSAR study and design of novel pyrazole derivatives as an anticancer lead compound against A-549, MCF-7, HeLa, HepG-2, PaCa-2, DLD-1 Computational Toxicology, 26: p. 100265
Elderdery AY et al (2022) Synthesis, Characterization, and Antiproliferative Effect of CuO-TiO 2-Chitosan-Amygdalin Nanocomposites in Human Leukemic MOLT4 Cells Bioinorganic Chemistry and Applications, 2022
Younas M et al (2023) Synthesis and characterization of cerium, silver and copper oxide nanoparticles and their anticancer potential of hepatocellular carcinoma HepG2 cancer cells. J Mol Struct 1288:135756
Bukhari A et al (2021) Green synthesis of metal and metal oxide nanoparticles using different plants’ parts for antimicrobial activity and anticancer activity: a review article. Coatings 11(11):1374
Lawrencia D et al (2021) Controlled release fertilizers: a review on coating materials and mechanism of release. Plants 10(2):238
Gopinath K, Sathishkumar G, Xu L (2024) An overview of the Copper Oxide Nanofillers Integrated in Food Packaging Systems. Coatings 14(1):81
Schaich KM, Tian X, Xie J (2015) Hurdles and pitfalls in measuring antioxidant efficacy: a critical evaluation of ABTS, DPPH, and ORAC assays. J Funct Foods 14:111–125
Thakur N et al (2024) A critical review on the recent trends of photocatalytic, antibacterial, antioxidant and nanohybrid applications of anatase and rutile TiO2 nanoparticles. Sci Total Environ, : p. 169815
Ma R et al (2019) Nanocomposite sponges of sodium alginate/graphene oxide/polyvinyl alcohol as potential wound dressing: in vitro and in vivo evaluation. Compos Part B: Eng 167:396–405
Acknowledgements
Not applicabe.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). No funds, grants, or other support was received.
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
Omnia Nemr and Zeinab S. Hamza: did the experimental work of the project.Mohamed Sh. Abdel-wahab and Hussein S. Mohamed: design and guide for the work.Ashraf A El-Bassuony: write the draft manuscript.Omayma F. Abdel Gawad: revised and final manusript.Sayed A. Ahmed: Supervisor.
Corresponding author
Ethics declarations
Ethical Approval
This article does not include any studies that use human or animal tissues.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nemr, O., Abdel-wahab, M., Hamza, Z. et al. Investigating the Anticancer and Antioxidant Potentials of a Polymer-Grafted Sodium Alginate Composite Embedded with CuO and TiO2 Nanoparticles. J Polym Environ 32, 2713–2728 (2024). https://doi.org/10.1007/s10924-024-03255-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-024-03255-z