Abstract
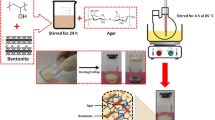
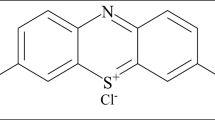
The wastewater treatment, particularly the removal of organic contaminants, is often carried out by adsorption with solids. Hydrogels present a great swelling capacity that can be enhanced by the incorporation of nano-clays such as montmorillonita (Mt). Methylene blue is a common component of industrial effluents and its ecotoxicity in well-known. The aim of this work was to evaluate the adsorption capacity of hydrogels based on pectin and brea gum with Mt nanoparticles. The hydrogels were physically characterized, it was observed that Mt is exfoliated into the matrix, and affects the swelling and erosion of them. Incorporation of Mt caused an increment of swelling of approximately 150% at pH 6.5. The effects of various experimental parameters have been investigated. The temperature effect on the adsorption rate indicated an endothermic process with activation energy of 33.231 kJ mol−1. The removal % of MB at 45 °C, pH 2.5 and 75 mg L−1 of MB solution presented a value of 97%. The adsorption kinetic was analysed through different mathematical models (pseudo-first order, pseudo-second order, intra-particle, and Boyd models). Statistics and further analysis of the kinetics constants indicated that the pseudo-second order model better represents the behaviour of the samples. The application of intra particle model and Boyd model indicates that the adsorption phenomenon occurs in two stages. The first stage, of short duration, is related to the boundary layer. The second stage, which controls the process, is related to the chemical adsorption of the dye on the polymeric structure of the hydrogel.









Similar content being viewed by others
References
Kausar A, Iqbal M, Javed A et al (2018) Dyes adsorption using clay and modified clay: a review. J Mol Liq 256:395–407. https://doi.org/10.1016/j.molliq.2018.02.034
Wang J, Guo X (2020) Adsorption kinetic models: physical meanings, applications, and solving methods. J Hazard Mater 390:122156. https://doi.org/10.1016/j.jhazmat.2020.122156
Pakdel PM, Peighambardoust SJ (2018) Review on recent progress in chitosan-based hydrogels for wastewater treatment application. Carbohydr Polym 201:264–279
Yagub MT, Sen TK, Afroze S, Ang HM (2014) Dye and its removal from aqueous solution by adsorption: a review. Adv Colloid Interface Sci 209:172–184. https://doi.org/10.1016/j.cis.2014.04.002
Slavutsky AM, Bertuzzi MA (2019) Formulation and characterization of hydrogel based on pectin and brea gum. Int J Biol Macromol 123:784–791. https://doi.org/10.1016/j.ijbiomac.2018.11.038
Maity J, Ray SK (2016) Enhanced adsorption of Cr(VI) from water by guar gum based composite hydrogels. Int J Biol Macromol 89:246–255. https://doi.org/10.1016/j.ijbiomac.2016.04.036
Ogata F, Ueta E, Kawasaki N (2016) Adsorption capability of ionic dyes onto pristine and calcined activated clay. e-J Surf Sci Nanotechnol 14:209–215. https://doi.org/10.1380/ejssnt.2016.209
Fiol N, Escudero C, Poch J, Villaescusa I (2006) Preliminary studies on Cr(VI) removal from aqueous solution using grape stalk wastes encapsulated in calcium alginate beads in a packed bed up-flow column. React Funct Polym 66:795–807. https://doi.org/10.1016/j.reactfunctpolym.2005.11.006
Xu D, Hein S, Loo SL, Wang K (2008) The fixed-bed study of dye removal on chitosan beads at high pH. Ind Eng Chem Res 47:8796–8800
Lazaridis NK, Keenan H (2010) Chitosan beads as barriers to the transport of azo dye in soil column. J Hazard Mater 173:144–150. https://doi.org/10.1016/j.jhazmat.2009.08.062
Lezehari M, Baudu M, Bouras O, Basly J (2012) Fixed-bed column studies of pentachlorophenol removal by use of alginate-encapsulated pillared clay microbeads. J Coll Interface Sci 379:101–106. https://doi.org/10.1016/j.jcis.2012.04.054
Vieira RM, Vilela PB, Becegato VA, Paulino AT (2018) Chitosan-based hydrogel and chitosan/acid-activated montmorillonite composite hydrogel for the adsorption and removal of Pb2+ and Ni2+ ions accommodated in aqueous solutions. J Environ Chem Eng 6:2713–2723. https://doi.org/10.1016/j.jece.2018.04.018
Aichour A, Zaghouane-boudiaf H, Binti F, Zuki M (2019) Low-cost, biodegradable and highly effective adsorbents for batch and column fixed bed adsorption processes of methylene blue. J Environ Chem Eng 7:103409. https://doi.org/10.1016/j.jece.2019.103409
Chen P-H, Kuo T-Y, Kuo J-Y et al (2010) Novel chitosan–pectin composite membranes with enhanced strength, hydrophilicity and controllable disintegration. Carbohydr Polym 82:1236–1242. https://doi.org/10.1016/j.carbpol.2010.06.057
Tan KL, Hameed BH (2017) Insight into the adsorption kinetics models for the removal of contaminants from aqueous solutions. J Taiwan Inst Chem Eng 74:25–48. https://doi.org/10.1016/j.jtice.2017.01.024
Piccin JS, Cadaval TRSA, De Pinto LAA, Dotto GL (2017) Adsorption isotherms in liquid phase: experimental, modeling, and interpretations. Springer, Cham
Anderson DR, Burnham KP, Press A (2008) When using information-theoretic pitfalls avoiding. Methods 66:912–918
Piolio MA, Slavutsky AMA, Koltan M et al (2019) Biopolymer-based formulations to improve the effect of the antimicrobial peptide Mcc J25(G12Y). Biocell 43(suppl 5):120
Abu Elella MH, Goda ES, Abdallah HM et al (2021) Innovative bactericidal adsorbents containing modified xanthan gum/montmorillonite nanocomposites for wastewater treatment. Int J Biol Macromol 167:1113–1125. https://doi.org/10.1016/j.ijbiomac.2020.11.065
Mekidiche M, Khaldi K, Nacer A, Boudjema S, Ameur N, Lerari-Zinai D, Bachari K, Choukchou-Braham A (2021) Organometallic modified montmorillonite application in the wastewater purification: Pollutant photodegradation and antibacterial efficiencies. Appl Surf Sci. https://doi.org/10.1016/j.apsusc.2021.151097
Bertuzzi MA, Slavutsky AM, Armada M (2012) Physicochemical characterisation of the hydrocolloid from Brea tree (Cercidium praecox). Int J Food Sci Technol 47:768–775. https://doi.org/10.1111/j.1365-2621.2011.02907.x
Castel V, Zivanovic S, Jurat-Fuentes JL et al (2016) Chromatographic fractionation and molecular mass characterization of Cercidium praecox (Brea) gum. J Sci Food Agric 96:4345–4350. https://doi.org/10.1002/jsfa.7642
Masuelli MA, Ochoa A (2018) Physicochemical parameters for brea gum exudate from Cercidium praecox tree. Coll Interface. https://doi.org/10.3390/colloids2040072
Sznaider F, Rojas AM, Stortz CA, Navarro DA (2020) Chemical structure and rheological studies of arabinoglucuronoxylans from the Cercidium praecox exudate brea gum. Carbohydr Polym 228:115388. https://doi.org/10.1016/j.carbpol.2019.115388
Weber WJ, Morris JC (1963) Kinetic of adsorption on carbon from solution. J Sanit Eng Div Proc Am Soc Civ Eng 89:31–60
Boyd GE, Schubert J, Adamson AW (1947) The exchange adsorption of ions from aqueous solutions by organic zeolites. I. Ion-Exchange Equilib 69:2818–2829
Reichenberg D (1953) Properties of ion-exchange resins in relation to their structure. III. Kinetics of exchange. J Am Chem Soc 75:589–597. https://doi.org/10.1021/ja01099a022
El-Khaiary MI, Malash GF (2011) Common data analysis errors in batch adsorption studies. Hydrometallurgy 105:314–320. https://doi.org/10.1016/j.hydromet.2010.11.005
Hurvich CM, Tsai CL (1989) Regression and time series model selection in small samples. Biometrika 76:297–307. https://doi.org/10.1093/biomet/76.2.297
Bozoğlan BK, Duman O, Tunç S (2020) Preparation and characterization of thermosensitive chitosan/carboxymethylcellulose/scleroglucan nanocomposite hydrogels. Int J Biol Macromol 162:781–797. https://doi.org/10.1016/j.ijbiomac.2020.06.087
Tang H, Zhou W, Zhang L (2012) Adsorption isotherms and kinetics studies of malachite green on chitin hydrogels. J Hazard Mater 209–210:218–225. https://doi.org/10.1016/j.jhazmat.2012.01.010
Slavutsky AM, Bertuzzi MA, Armada M, García MG, Ochoa NA (2014) Preparation and characterization of montmorillonite/brea gum nanocomposites films. Food Hydrocoll 35:270–278
Cabello SDP, Takara EA, Marchese J, Ochoa NA (2015) Influence of plasticizers in pectin fi lms: microstructural changes. Mater Chem Phys. https://doi.org/10.1016/j.matchemphys.2015.06.019
Gorrasi G, Bugatti V, Viscusi G, Vittoria V (2021) Physical and barrier properties of chemically modified pectin with polycaprolactone through an environmentally friendly process. Colloid Polym Sci 299:429–437. https://doi.org/10.1007/s00396-020-04699-0
Fan T, Liu Z, Zhao Y et al (2022) Synthesis of a tough montmorillonite/hydrogel composites for hot work of long-distance oil pipelines. Compos Sci Technol. https://doi.org/10.1016/j.compscitech.2022.109268
Müller CMO, Borges J, Yamashita F (2011) Effect of nanoclay incorporation method on mechanical and water vapor barrier properties of starch-based films. Ind Crops Prod 33:605–610. https://doi.org/10.1016/j.indcrop.2010.12.021
Jafari H, Atlasi Z, Mahdavinia GR et al (2021) Magnetic κ-carrageenan/chitosan/montmorillonite nanocomposite hydrogels with controlled sunitinib release. Mater Sci Eng C 124:112042. https://doi.org/10.1016/j.msec.2021.112042
Qian JY, Chen W, Zhang WM, Zhang H (2009) Adulteration identification of some fungal polysaccharides with SEM, XRD, IR and optical rotation: a primary approach. Carbohydr Polym 78:620–625. https://doi.org/10.1016/j.carbpol.2009.05.025
Slavutsky AM, Bertuzzi MA, Armada M et al (2014) Preparation and characterization of montmorillonite/brea gum nanocomposites films. Food Hydrocoll. https://doi.org/10.1016/j.foodhyd.2013.06.008
Chomto P, Nunthanid J (2017) Physicochemical and powder characteristics of various citrus pectins and their application for oral pharmaceutical tablets. Carbohydr Polym 174:25–31. https://doi.org/10.1016/j.carbpol.2017.06.049
Bigucci F, Luppi B, Cerchiara T et al (2008) Chitosan/pectin polyelectrolyte complexes: selection of suitable preparative conditions for colon-specific delivery of vancomycin. Eur J Pharm Sci 35:435–441. https://doi.org/10.1016/j.ejps.2008.09.004
Naidu VGM, Madhusudhana K, Sashidhar RB et al (2009) Polyelectrolyte complexes of gum kondagogu and chitosan, as diclofenac carriers. Carbohydr Polym 76:464–471. https://doi.org/10.1016/j.carbpol.2008.11.010
Wang W, Zhao Y, Yi H et al (2019) Pb(ΙΙ) removal from water using porous hydrogel of chitosan-2D montmorillonite. Int J Biol Macromol 128:85–93. https://doi.org/10.1016/j.ijbiomac.2019.01.098
Wang J, Wang W, Ai Z et al (2021) Adsorption toward Pb(II) occurring on three-dimensional reticular-structured montmorillonite hydrogel surface. Appl Clay Sci 210:106153. https://doi.org/10.1016/j.clay.2021.106153
Ray SS, Bousmina M (2005) Biodegradable polymers and their layered silicate nanocomposites: in greening the 21st century materials world. Prog Mater Sci 50:962–1079. https://doi.org/10.1016/j.pmatsci.2005.05.002
Bhattacharyya KG, SenGupta S, Sarma GK (2014) Interactions of the dye, Rhodamine B with kaolinite and montmorillonite in water. Appl Clay Sci 99:7–17. https://doi.org/10.1016/j.clay.2014.07.012
Peng N, Hu D, Zeng J et al (2016) Superabsorbent cellulose-clay nanocomposite hydrogels for highly efficient removal of dye in water. ACS Sustain Chem Eng 4:7217–7224. https://doi.org/10.1021/acssuschemeng.6b02178
Dai H, Huang Y, Huang H (2018) Eco-friendly polyvinyl alcohol/carboxymethyl cellulose hydrogels reinforced with graphene oxide and bentonite for enhanced adsorption of methylene blue. Carbohydr Polym 185:1–11. https://doi.org/10.1016/j.carbpol.2017.12.073
da Costa MPM, Ferreira ILM, Cruz MTM (2016) New polyelectrolyte complex from pectin/chitosan and montmorillonite clay. Carbohydr Polym 146:123–130. https://doi.org/10.1016/j.carbpol.2016.03.025
Bauli CR, Lima GF, de Souza AG et al (2021) Eco-friendly carboxymethyl cellulose hydrogels filled with nanocellulose or nanoclays for agriculture applications as soil conditioning and nutrient carrier and their impact on cucumber growing. Colloids Surf A 623:126771. https://doi.org/10.1016/j.colsurfa.2021.126771
Wang W, Zhao Y, Bai H et al (2018) Methylene blue removal from water using the hydrogel beads of poly(vinyl alcohol)-sodium alginate-chitosan-montmorillonite. Carbohydr Polym 198:518–528. https://doi.org/10.1016/j.carbpol.2018.06.124
Felycia SI, Soetaredjo E, Ayucitra A (2015) Clay materials for environmental remediation. Springer, Cham
Çelik MS (2004) Electrokinetic behavior of clay surfaces. Elsevier, Amsterdam
Bello MO, Abdus-Salam N, Adekola FA, Pal U (2021) Isotherm and kinetic studies of adsorption of methylene blue using activated carbon from ackee apple pods. Chem Data Collect 31:100607. https://doi.org/10.1016/j.cdc.2020.100607
Li Y, Du Q, Liu T et al (2013) Comparative study of methylene blue dye adsorption onto activated carbon, graphene oxide, and carbon nanotubes. Chem Eng Res Des 91:361–368. https://doi.org/10.1016/j.cherd.2012.07.007
Abu Elella MH, Goda ES, Gamal H et al (2021) Green antimicrobial adsorbent containing grafted xanthan gum/SiO2 nanocomposites for malachite green dye. Int J Biol Macromol 191:385–395. https://doi.org/10.1016/j.ijbiomac.2021.09.040
Nethaji S, Sivasamy A (2011) Adsorptive removal of an acid dye by lignocellulosic waste biomass activated carbon: Equilibrium and kinetic studies. Chemosphere 82:1367–1372. https://doi.org/10.1016/j.chemosphere.2010.11.080
Nethaji S, Sivasamy A, Thennarasu G, Saravanan S (2010) Adsorption of Malachite Green dye onto activated carbon derived from Borassus aethiopum flower biomass. J Hazard Mater 181:271–280. https://doi.org/10.1016/j.jhazmat.2010.05.008
Karmaker S, Sintaha F, Saha TK (2019) Kinetics, isotherm and thermodynamic studies of the adsorption of reactive red 239 dye from aqueous solution by chitosan 8B. Adv Biol Chem 09:1–22. https://doi.org/10.4236/abc.2019.91001
Pholosi A, Naidoo EB, Ofomaja AE (2020) Intraparticle diffusion of Cr(VI) through biomass and magnetite coated biomass: a comparative kinetic and diffusion study. S Afr J Chem Eng 32:39–55. https://doi.org/10.1016/j.sajce.2020.01.005
Zulfikar MA, Setiyanto H, Djajanti SD (2013) Effect of temperature and kinetic modelling of lignosulfonate adsorption onto powdered eggshell in batch systems. Songklanakarin J Sci Technol 35:309–316
Mahmoud DK, Salleh MAM, Karim WAWA et al (2012) Batch adsorption of basic dye using acid treated kenaf fibre char: equilibrium, kinetic and thermodynamic studies. Chem Eng J 181–182:449–457. https://doi.org/10.1016/j.cej.2011.11.116
Ma J, Jia Y, Jing Y et al (2012) Kinetics and thermodynamics of methylene blue adsorption by cobalt-hectorite composite. Dye Pigment 93:1441–1446. https://doi.org/10.1016/j.dyepig.2011.08.010
Plazinski W, Rudzinski W, Plazinska A (2009) Theoretical models of sorption kinetics including a surface reaction mechanism: a review. Adv Colloid Interface Sci 152:2–13. https://doi.org/10.1016/j.cis.2009.07.009
García ER, Medina RL, Lozano MM et al (2014) Adsorption of azo-dye orange II from aqueous solutions using a metal-organic framework material: iron-benzenetricarboxylate. Materials (Basel) 7:8037–8057. https://doi.org/10.3390/ma7128037
Benhouria A, Islam MA, Zaghouane-Boudiaf H et al (2015) Calcium alginate-bentonite-activated carbon composite beads as highly effective adsorbent for methylene blue. Chem Eng J 270:621–630. https://doi.org/10.1016/j.cej.2015.02.030
Azizian S (2004) Kinetic models of sorption: a theoretical analysis. J Colloid Interface Sci 276:47–52. https://doi.org/10.1016/j.jcis.2004.03.048
Malash GF, El-Khaiary MI (2010) Piecewise linear regression: a statistical method for the analysis of experimental adsorption data by the intraparticle-diffusion models. Chem Eng J 163:256–263. https://doi.org/10.1016/j.cej.2010.07.059
Tran HN, You SJ, Chao HP (2016) Thermodynamic parameters of cadmium adsorption onto orange peel calculated from various methods: a comparison study. J Environ Chem Eng 4:2671–2682. https://doi.org/10.1016/j.jece.2016.05.009
Thakur S, Pandey S, Arotiba OA (2016) Development of a sodium alginate-based organic/inorganic superabsorbent composite hydrogel for adsorption of methylene blue. Carbohydr Polym. https://doi.org/10.1016/j.carbpol.2016.06.104
Al-Ghouti M, Khraisheh MAM, Ahmad MNM, Allen S (2005) Thermodynamic behaviour and the effect of temperature on the removal of dyes from aqueous solution using modified diatomite: a kinetic study. J Colloid Interface Sci 287:6–13. https://doi.org/10.1016/j.jcis.2005.02.002
Acknowledgements
The authors thank the staff of LASEM (Laboratorio de Microscopia Electrónica de Barrido, ANPCyT, CONICET, UNSA) technical assistance and Dr. Javier Molla for the XRD analysis (UCASAL).
Funding
The financial support provided by Consejo de Investigación de la Universidad Nacional de Salta (CIUNSa-Proyecto 2617) and Agencia Nacional Científica y Tecnológica (PICT 2019-2704) are gratefully acknowledged.
Author information
Authors and Affiliations
Contributions
All authors contributed to conception and design of the study. Material preparation, data collection, and analysis were performed by AMS. The first draft of the manuscript was written by AMS and all authors commented and contributed to subsequent versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gamboni, J.E., Bertuzzi, M.A. & Slavutsky, A.M. Methylene Blue Sorption Phenomena onto Pectin, Brea Gum, Montmorillonite Based Hydrogels: Kinetic and Thermodynamic Assessment. J Polym Environ 30, 4710–4725 (2022). https://doi.org/10.1007/s10924-022-02546-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-022-02546-7