Abstract
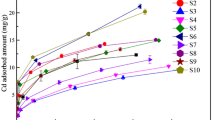
Many industrial wastewaters are contaminated with both heavy metal ions and organic compounds, posing a major threat to public health and the ecosystem. In this work, pristine chitosan modified vermiculite (CSVT) with high specific surface area (17.24 m2/g) was prepared by drop-wise method and applied to simultaneous adsorption of Pb(II) and phenol from aqueous solution. The prepared adsorbents were characterized by X-ray diffraction, Brunauer, Emmett, Teller, energy dispersive X-ray spectroscopy, Fourier-transform infrared, and scanning electron microscopy. The simultaneous adsorption performance was investigated systematically at different solution-pH, adsorbent dosage, initial Pb(II) and phenol concentrations, and contact time. Kinetic experiments indicated that the adsorption process of both pollutants could be better represented by a pseudo-second-order model. The maximal adsorption capacities of Pb(II) and phenol in the binary system depending on Langmuir isotherm model are 98.52 and 67.08 mg/g, respectively. In the binary adsorption system, the adsorption of one contaminant was not affected by the other adsorbed one. Finally, the results clearly showed that chitosan modified vermiculite had the ability to simultaneously adsorb Pb(II) and phenol.












Similar content being viewed by others
References
Li N, Bai R (2006) Highly enhanced adsorption of lead ions on chitosan granules functionalized with poly(acrylic acid). Ind Eng Chem Res 45(23):7897–7904
Salih SS, Ghosh TK (2018) Highly efficient competitive removal of Pb(II) and Ni(II) by chitosan/diatomaceous earth composite. J Environ Chem Eng 6(1):435–443
Arcibar-Orozco JA, Rangel-Mendez JR, Diaz-Flores PE (2015) Simultaneous adsorption of Pb(Ii)-Cd(Ii), Pb(Ii)-phenol, and Cd(Ii)-phenol by activated carbon cloth in aqueous solution. Water Air Soil Pollut 226(1):1–10
Istratie R, Stoia M, Păcurariu C, Locovei C (2019) Single and simultaneous adsorption of methyl orange and phenol onto magnetic iron oxide/carbon nanocomposites. Arab J Chem 12(8):3704–3722
Wang P, Tyndall S, Rahman T, Roy P, Jahromi H, Adhikari S, Boersma M (2022) Sorption and recovery of phenolic compounds from aqueous phase of sewage sludge hydrothermal liquefaction using bio-char. Chemosphere 287:131934
Chakraborty R, Asthana A, Singh AK, Jain B, Susan ABH (2022) Adsorption of heavy metal ions by various low-cost adsorbents: a review. Int J Environ Anal Chem 102(2):342–379
Salih SS, Ghosh TK (2017) Preparation and characterization of bioadsorbent beads for chromium and zinc ions adsorption. Cogent Environ Sci 3(1):1401577
Saleh TA, Tuzen M, Sarı A, Altunay N (2022) Factorial design, physical studies and rapid arsenic adsorption using newly prepared polymer modified perlite adsorbent. Chem Eng Res Des. https://doi.org/10.1016/j.cherd.2022.04.042
Tuzen M (2019) Development of tetraethylene pentamine functionalized multi-wall carbon nanotubes as a new adsorbent in a syringe system for removal of bisphenol A by using multivariate optimization techniques. Microchem J 147:1147–1154
Pouya ES, Abolghasemi H, Esmaieli M, Fatoorehchi H, Hashemi SJ, Salehpour A (2015) Batch adsorptive removal of benzoic acid from aqueous solution onto modified natural vermiculite: kinetic, isotherm and thermodynamic studies. J Ind Eng Chem 31:199–215
Salih SS, Ghosh TK (2018) Preparation and characterization of chitosan-coated diatomaceous earth for hexavalent chromium removal. Environ Process 5(1):23–39
Du Z, Deng S, Zhang S, Wang W, Wang B, Huang J, Xing B (2017) Selective and fast adsorption of perfluorooctanesulfonate from wastewater by magnetic fluorinated vermiculite. Environ Sci Technol 51(14):8027–8035
Salih SS, Mahdi A, Kadhom M, Ghosh TK (2019) Competitive adsorption of As(III) and As(V) onto chitosan/diatomaceous earth adsorbent. J Environ Chem Eng 7(5):103407
Saleh TA, Sarı A, Tuzen M (2019) Carbon nanotubes grafted with poly(trimesoyl, m-phenylenediamine) for enhanced removal of phenol. J Environ Manag 252:109660
dos Anjos VE, Rohwedder JR, Cadore S, Abate G, Grassi MT (2014) Montmorillonite and vermiculite as solid phases for the preconcentration of trace elements in natural waters: adsorption and desorption studies of As, Ba, Cu, Cd Co, Cr, Mn, Ni, Pb, Sr, V, and Zn. Appl Clay Sci 99:289–296
Vijayaraghavan K, Raja FD (2015) Interaction of vermiculite with Pb(II), Cd(II), Cu(II) and Ni(II) ions in single and quaternary mixtures. Clean: Soil, Air, Water 43(8):1174–1180
Mazloomi F, Jalali M (2017) Adsorption of ammonium from simulated wastewater by montmorillonite nanoclay and natural vermiculite: experimental study and simulation. Environ Monit Assess 189(8):1–19
Stawiński W, Węgrzyn A, Freitas O, Chmielarz L, Mordarski G, Figueiredo S (2017) Simultaneous removal of dyes and metal cations using an acid, acid–base and base modified vermiculite as a sustainable and recyclable adsorbent. Sci Total Environ 576:398–408
Hashem FS, Amin MS, El-Gamal SMA (2015) Chemical activation of vermiculite to produce highly efficient material for Pb2+ and Cd2+ removal. Appl Clay Sci 115:189–200
Zhao F, Repo E, Yin D, Sillanpää ME (2013) Adsorption of Cd(II) and Pb(II) by a novel EGTA-modified chitosan material: kinetics and isotherms. J Colloid Interface Sci 409:174–182
Saleh TA, Sarı A, Tuzen M (2016) Chitosan-modified vermiculite for As(III) adsorption from aqueous solution: equilibrium, thermodynamic and kinetic studies. J Mol Liq 219:937–945
Hande PE, Kamble S, Samui AB, Kulkarni PS (2016) Chitosan-based lead ion-imprinted interpenetrating polymer network by simultaneous polymerization for selective extraction of lead(II). Ind Eng Chem Res 55(12):3668–3678
de Araújo EL, Barbosa HFG, Dockal ER, Cavalheiro ÉTG (2017) Synthesis, characterization and biological activity of Cu(II), Ni(II) and Zn(II) complexes of biopolymeric Schiff bases of salicylaldehydes and chitosan. Int J Biol Macromol 95:168–176
Pourbeyram S (2016) Effective removal of heavy metals from aqueous solutions by graphene oxide–zirconium phosphate (GO–Zr-P) nanocomposite. Ind Eng Chem Res 55(19):5608–5617
Osorio-Madrazo A, David L, Trombotto S, Lucas JM, Peniche-Covas C, Domard A (2010) Kinetics study of the solid-state acid hydrolysis of chitosan: evolution of the crystallinity and macromolecular structure. Biomacromol 11(5):1376–1386
Li K, Bian S, Zhen W, Li H, Zhao L (2021) Performance, crystallization and rheological behavior of poly(lactic acid)/N-(2-hydroxyl) propyl-3-trimethyl ammonium chitosan chloride intercalated vermiculite grafted poly (acrylamide) nanocomposites. React Funct Polym 158:104791
Salih SS, Mohammed HN, Abdullah GH, Kadhom M, Ghosh TK (2020) Simultaneous removal of Cu(II), Cd(II), and industrial dye onto a composite chitosan biosorbent. J Polym Environ 28(1):354–365
Padilla-Ortega E, Leyva-Ramos R, Mendoza-Barron J (2014) Role of electrostatic interactions in the adsorption of cadmium(II) from aqueous solution onto vermiculite. Appl Clay Sci 88:10–17
Kumari HJ, Krishnamoorthy P, Arumugam TK, Radhakrishnan S, Vasudevan D (2017) An efficient removal of crystal violet dye from waste water by adsorption onto TLAC/chitosan composite: a novel low cost adsorbent. Int J Biol Macromol 96:324–333
Abdel-Ghani NT, El-Chaghaby GA, Helal FS (2015) Individual and competitive adsorption of phenol and nickel onto multiwalled carbon nanotubes. J Adv Res 6(3):405–415
Alizadeh B, Delnavaz M, Shakeri A (2018) Removal of Cd(ӀӀ) and phenol using novel cross-linked magnetic EDTA/chitosan/TiO2 nanocomposite. Carbohydr Polym 181:675–683
Asmaly HA, Ihsanullah AB, Saleh TA, Laoui T, Gupta VK, Atieh MA (2016) Adsorption of phenol on aluminum oxide impregnated fly ash. Desalin Water Treat 57(15):6801–6808
Wang N, Xu X, Li H, Yuan L, Yu H (2016) Enhanced selective adsorption of Pb(II) from aqueous solutions by one-pot synthesis of xanthate-modified chitosan sponge: behaviors and mechanisms. Ind Eng Chem Res 55(47):12222–12231
Wang G, Zhang S, Hua Y, Su X, Ma S, Wang J, Komarneni S (2017) Phenol and/or Zn2+ adsorption by single-or dual-cation organomontmorillonites. Appl Clay Sci 140:1–9
Singh DK, Kumar V, Mohan S, Hasan SH (2017) Polylysine functionalized graphene aerogel for the enhanced removal of Cr(VI) through adsorption: kinetic, isotherm, and thermodynamic modeling of the process. J Chem Eng Data 62(5):1732–1742
Şenol ZM, Şimşek S (2020) Equilibrium, kinetics and thermodynamics of Pb(II) ions from aqueous solution by adsorption onto chitosan-dolomite composite beads. Int J Environ Anal Chem. https://doi.org/10.1080/03067319.2020.1790546
Xu J, Liu X, Lowry GV, Cao Z, Zhao H, Zhou JL, Xu X (2016) Dechlorination mechanism of 2,4-dichlorophenol by magnetic MWCNTs supported Pd/Fe nanohybrids: rapid adsorption, gradual dechlorination, and desorption of phenol. ACS Appl Mater Interfaces 8(11):7333–7342
Tran L, Wu P, Zhu Y, Liu S, Zhu N (2015) Comparative study of Hg(II) adsorption by thiol-and hydroxyl-containing bifunctional montmorillonite and vermiculite. Appl Surf Sci 356:91–101
Şenol ZM (2021) A chitosan-based composite for adsorption of uranyl ions; mechanism, isotherms, kinetics and thermodynamics. Int J Biol Macromol 183:1640–1648
Şenol ZM, Şimşek S (2022) Insights into effective adsorption of lead ions from aqueous solutions by using chitosan-bentonite composite beads. J Polym Environ. https://doi.org/10.1007/s10924-022-02464-8
Liu S, Wu P, Yu L, Li L, Gong B, Zhu N, Yang C (2017) Preparation and characterization of organo-vermiculite based on phosphatidylcholine and adsorption of two typical antibiotics. Appl Clay Sci 137:160–167
Saleh TA, Sarı A, Tuzen M (2017) Effective adsorption of antimony(III) from aqueous solutions by polyamide-graphene composite as a novel adsorbent. Chem Eng J 307:230–238
Şenol ZM, Gürsoy N, Şimşek S, Özer A, Karakuş N (2020) Removal of food dyes from aqueous solution by chitosan-vermiculite beads. Int J Biol Macromol 148:635–646
Li X, Wang S, Liu Y, Jiang L, Song B, Li M, Ding Y (2017) Adsorption of Cu(II), Pb(II), and Cd(II) ions from acidic aqueous solutions by diethylenetriaminepentaacetic acid-modified magnetic graphene oxide. J Chem Eng Data 62(1):407–416
Şenol ZM, Şimşek S, Özer A, Şenol Arslan D (2021) Synthesis and characterization of chitosan–vermiculite composite beads for removal of uranyl ions: isotherm, kinetics and thermodynamics studies. J Radioanal Nucl Chem 327(1):159–173
Şimşek S, Kaya S, Şenol ZM, Ulusoy Hİ, Katin KP, Özer A, Brahmia A (2022) Theoretical and experimental insights about the adsorption of uranyl ion on a new designed vermiculite-polymer composite. J Mol Liq 352:118727
Şenol ZM, Kaya S, Şimşek S, Katin KP, Özer A, Marzouki R (2022) Synthesis and characterization of chitosan-vermiculite-lignin ternary composite as an adsorbent for effective removal of uranyl ions from aqueous solution: experimental and theoretical analyses. Int J Biol Macromol 209:1234–1247
Cheng WP, Gao W, Cui X, Ma JH, Li RF (2016) Phenol adsorption equilibrium and kinetics on zeolite X/activated carbon composite. J Taiwan Inst Chem Eng 62:192–198
Ma L, Chen Q, Zhu J, Xi Y, He H, Zhu R, Ayoko GA (2016) Adsorption of phenol and Cu(II) onto cationic and zwitterionic surfactant modified montmorillonite in single and binary systems. Chem Eng J 283:880–888
Wang Y, Li L, Luo C, Wang X, Duan H (2016) Removal of Pb2+ from water environment using a novel magnetic chitosan/graphene oxide imprinted Pb2+. Int J Biol Macromol 86:505–511
Ngah WW, Fatinathan S (2010) Adsorption characterization of Pb(II) and Cu(II) ions onto chitosan-tripolyphosphate beads: kinetic, equilibrium and thermodynamic studies. J Environ Manag 91(4):958–969
do Nascimento FH, de Souza Costa DM, Masini JC (2016) Evaluation of thiol-modified vermiculite for removal of Hg(II) from aqueous solutions. Appl Clay Sci 124:227–235
Huang Y, Fulton AN, Keller AA (2016) Simultaneous removal of PAHs and metal contaminants from water using magnetic nanoparticle adsorbents. Sci Total Environ 571:1029–1036
Lin SH, Juang RS (2009) Adsorption of phenol and its derivatives from water using synthetic resins and low-cost natural adsorbents: a review. J Environ Manag 90(3):1336–1349
Acknowledgements
The authors would like to thank the Nuclear Science and Engineering Institute at the University of Missouri-Columbia, USA for allowing us working in their laboratories. In addition, the authors thank the Chemical Engineering Department at the University of Tikrit, Iraq for financial support of the experimental studies.
Funding
The authors have not disclosed any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing Interests
The authors have not disclosed any competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Salih, S.S., Kadhom, M., Shihab, M.A. et al. Competitive Adsorption of Pb(II) and Phenol Onto Modified Chitosan/Vermiculite Adsorbents. J Polym Environ 30, 4238–4251 (2022). https://doi.org/10.1007/s10924-022-02515-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-022-02515-0