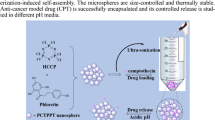
Abstract
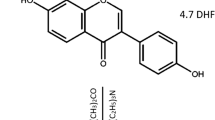
Cross-linked polyphosphazene-based microspheres have great promise for their specific properties and uniform structures and have attracted much attention due to their potential applications. In this research, cross-linked poly(cyclotriphosphazene-co-fluorescein) (PCTPF) microspheres were synthesized and used as carriers for drug delivery applications. The successful preparation of the PCTPF microspheres was confirmed by Fourier transform infrared (FT-IR) spectroscopy and scanning electron microscope (SEM). The new appeared bands in FT-IR spectra confirmed the successful polymerization between hexachlorocyclotriphosphazene (HCCP) and fluorescein (FLUS). While the SEM results showed that the size of the microspheres was strongly dependent on the mole ratio of HCCP to FLUS. The particle size distribution was determined by dynamic light scattering (DLS) and the results showed that the decreased mole ratio of HCCP to FLUS increases the size of the microspheres. The crystalline nature of PCTPF microspheres was checked by X-ray diffraction and the results showed the amorphous nature of PCTPF microspheres. The thermal properties of the PCTPF microspheres were examined by thermogravimetric analysis and the results exhibited that the increase in thermal properties was ascribed to the cross-linked covalently bonded structure of PCTPF microspheres. The cross-linking density of the prepared PCTPF microspheres was examined via electron paramagnetic resonance (EPR) spectroscopy and the obtained results suggested that the decreased HCCP:FLUS mole ratio could be more capable to cross-link with HCCP in high density. The prepared PCTPF-3 microspheres were used as drug carriers for the anti-cancer drug “Fluorouracil” (5-FU). 5-FU drug was loaded on the prepared microspheres and their in vitro drug release experiment was performed, in which the results showed that the drug released from the PCTPF-3/5-FU microspheres was in a controlled manner up to 336 h. According to the results, the PCTPF microspheres might be used in cancer therapy as drug carriers.
Graphical Abstract












Similar content being viewed by others
References
Jong WH, Borm P (2008) Drug delivery and nanoparticles: Applications and hazards. Int J Nanomed 3:133–149
Prabhakar U, Maeda H, Jain RK, Sevick M, Eva M, Zamboni W, Farokhzad OC (2013) Challenges and key considerations of the enhanced permeability and retention effect for nanomedicine drug delivery in oncology. Cancer Res 73:2412–2417
Doane TL, Burda C (2012) The unique role of nanoparticles in nanomedicine: Imaging, drug delivery and therapy. Chem Soc Rev 41:2885–2911
Santosh S, Rajagopalan MD, Pallavi BA, Rudramurthy GR, Rajashekar V, Sridhar KA (2017) Cancer therapies: Current scenario, management, and safety aspects. Anticancer Plants Clinical Trials Nanotechnol 3:1–25
Khor SY, Quinn JF, Whittaker MR, Truong NP, Davis TP (2019) Controlling nanomaterial size and shape for biomedical applications via polymerization-induced self-assembly. Macromol Rapid Commun 40:1800438
Qian H, Liu B, Jiang X (2018) Application of nanomaterials in cancer immunotherapy. Mater Today Chem 7:53–64
Cheng F, Peng X, Meng G, Pu Y (2020) Poly(ester-thioether) microspheres co-loaded with erlotinib and α-tocopheryl succinate for combinational therapy of non-small cell lung cancer. J Mater Chem B 8:1728–1738
Varde NK, Pack DW (2004) Microspheres for controlled release drug delivery. Expert Opin Biol Ther 4:35–51
Acerbi F, Cavallo C, Broggi M, Cordella R, Anghileri E, Eoli M (2014) Fluorescein-guided surgery for malignant gliomas: A review. Neurosurg Rev 37:547–557
Sun L, Liu T, Li H, Yang L, Meng L, Lu Q (2015) Fluorescent and cross-linked organic-inorganic hybrid nanoshells for monitoring drug delivery. ACS Appl Mater Interfaces 7:4990–4997
Allcock HR (2004) The crucial role of inorganic ring chemistry in the development of new polymers. Phosphorus Sulfur Silicon Relat Elem 179:661–671
Kim D, Geun WS, Kim D, Kwan PS, Han HJ, Taek, (2005) Fabrication and characteristics of plastic optical fiber directional couplers. J Opt Soc Korea 9:99–102
Allcock HR, Kellam EC, Hofmann MA (2001) Synthesis of cyclolinear phosphazene-containing polymers via admet polymerization. Macromolecules 34:5140–5146
Ali S, Zuhra Z, Butler IS, Dar SU, Hameed MU, Wu D (2017) High-throughput synthesis of cross-linked poly(cyclotriphosphazene-co-bis (aminomethyl) ferrocene) microspheres and their performance as a superparamagnetic, electrochemical, fluorescent and adsorbent material. Chem Eng J 315:448–458
Abbas Y, Zuhra Z, Wu Z, Wu D, Ali S (2019) Poly(ferrocenedimethano)-cyclotriphosphazene to homogenously Fe, N, P, O doped carbon nanotubes: An efficient and tremendous electrocatalyst for oxygen reduction reaction. J Electrochem Soc 166:297–303
Abbas Y, Basharat M, Liu W, Khan SM, Zhang S, Ali S (2020) Substantial role of nitrogen and sulfur in quaternary-atom-doped multishelled carbon nanospheres for the oxygen evolution reaction. ACS Sustain Chem Eng 8:4284–4291
Basharat M, Liu W, Zhang S, Abbas Y, Wu Z, Wu D (2019) Poly(cyclotriphosphazene-co-tris(4-hydroxyphenyl)ethane) microspheres with intrinsic excitation wavelengthtunable multicolor photoluminescence. Macromol Chem Phys 220:1900256
Wei W, Huang X, Chen K, Tao Y, Tang X (2012) Fluorescent organic-inorganic hybrid polyphosphazene microspheres for the trace detection of nitroaromatic explosives. RSC Adv 2:3765–3771
Wei W, Huang X, Zhao X, Zhang P, Tang X (2010) A rapid and efficient strategy for preparation of super-hydrophobic surface with cross-linked cyclotriphosphazene/6F-bisphenol A copolymer microspheres. Chem Commun 46:487–489
Pan T, Huang X, Wei H, Wei W, Tang X (2012) Intrinsically fluorescent microspheres with superior thermal stability and broad ultraviolet-visible absorption based on hybrid polyphosphazene material. Macromol Chem Phys 213:1590–1595
Zhu L, Zhu Y, Pan Y, Huang Y, Huang X, Tang X (2007) Fully cross-linked poly[cyclotriphosphazene-co-(4,4′-sulfonyldiphenol)] microspheres via precipitation polymerization and their superior thermal properties. Macromol React Eng 1:45–52
Metinoğlu ÖS, Süzen DY (2019) One-pot synthesis and characterization of cross-linked polyphosphazene dopamine microspheres for controlled drug delivery applications. J Macromol Sci Part A 56:854–859
Hou S, Zhou S, Chen S, Lu Q (2020) Polyphosphazene-based drug self-framed delivery system as a universal intelligent platform for combination therapy against multidrug-resistant tumors. ACS Appl Bio Mater 3:2284–2294
Fu J, Chen Z, Wu X, Wang M, Wang X, Zhang J (2015) Hollow poly(cyclotriphosphazene-co-phloroglucinol) microspheres: An effective and selective adsorbent for the removal of cationic dyes from aqueous solution. Chem Eng J 281:42–52
Hong S, Li J, Huang X, Liu H (2018) A facile approach to generate cross-linked poly(cyclotriphosphazene-co-oxyresveratrol) nanoparticle with intrinsically fluorescence. J Inorg Organomet Polym Mater 28:2258–2263
Uddin MA, Yu H, Wang L, K-u-R N, Haq F, Amin BU (2020) Recent progress in EPR study of spin labeled polymers and spin probed polymer systems. J Polym Sci 58:1924–1948
Constantin MM, Corbu CG, Tanase C, Codrici E, Mihai S, Popescu ID (2019) Spin probe method of electron paramagnetic resonance spectroscopy-a qualitative test for measuring the evolution of dry eye syndrome under treatment. Anal Methods 11:965–792
Örüm SM, Demircioğlu YS (2018) Cross-linked polyphosphazene nanospheres with anticancer quercetin: Synthesis, spectroscopic, thermal properties, and controlled drug release. Macromol Res 26:671–679
Karuppuswamy P, Reddy Venugopal J, Navaneethan B, Luwang Laiva A, Ramakrishna S (2015) Polycaprolactone nanofibers for the controlled release of tetracycline hydrochloride. Mater Lett 141:180–186
Eun Shim S, Yang S, Choe S (2004) Mechanism of the formation of stable microspheres by precipitation copolymerization of styrene and divinylbenzene. J Polym Sci Part A Polym Chem 42:3967–3974
Wang Y, Mu J, Li L, Shi L, Zhang W, Jiang Z (2012) Preparation and properties of novel fluorinated cross-linked polyphosphazene micro-nano spheres. High Perform Polym 24:229–236
Abbas Y, Zuhra Z, Basharat M, Qiu M, Wu Z, Wu D (2019) Morphology Control of novel cross-linked ferrocenedimethanol derivative cyclophosphazenes: From microspheres to nanotubes and their enhanced physicochemical performances. J Phys Chem B 123:4148–4156
Abbas Y, Ali S, Basharat M, Zou W, Yang F, Liu W (2020) Heteroatom-doped carbon nanoparticle-ionic liquid composites as electrochemical sensors for uric acid. ACS Appl Nano Mater 3:11383–11390
Basharat M, Abbas Y, Liu W, Ali Z, Zhang S, Zou W (2019) Unusual excitation wavelength tunable multiple fluorescence from organocyclophosphazene microspheres: Cross-linked structure-property relationship. Polymer 185:121942
Wang L, Roitberg A, Meuse C, Gaigalas AK (2001) Raman and FTIR spectroscopies of fluorescein in solutions. Spectrochim Acta Part A Mol Biomol Spec 1781–1791
Wei X, Zhang G, Zhou L, Li J (2017) Synthesis and characterization of hydrophobic amino-based polyphosphazene microspheres with different morphologies via two strategies. Appl Surf Sci 419:744–752
Uddin MA, Yu H, Wang L, Amin BU, Mehmood S, Liang R (2021) Dynamics in controllable stimuli-responsive self-assembly of polymer vesicles with stable radical functionality. ACS Appl Mater Interfaces 31:61693
Uddin MA, Yu H, Wang L, Sheng Y, Mehmood S, Amin BU (2021) Stimuli-sensitivity and dynamics in the self-assembly structure of TEMPO-containing nanoparticles and their triggering hydrophobic drug release. Mater Today Commun 30:103107
Uddin MA, Yu H, Wang L, K-u-R N, Amin BU, Mehmood S (2021) Multiple-stimuli-responsiveness and conformational inversion of smart supramolecular nanoparticles assembled from spin labeled amphiphilic random copolymers. J Colloid Interface Sci 585:237–249
Uddin MA, Yu H, Wang L, Liu J, Mehmood S, Amin BU (2021) Multi-stimuli-responsive performance and morphological changes of radical-functionalized self-assembled micellar nanoaggregates and their multi-triggered drug release. Colloids Surf A Physicochem Eng Asp 625:126807
Li H, Zhang B, Lü S, Ma H, Liu M (2018) Synthesis and characterization of a nano fluorescent starch. Int J Biol Macromol 120:1225–1231
Xu W, Park JY, Kattel K, Ahmad MW, Bony BA, Heo WC (2012) Fluorescein-polyethyleneimine coated gadolinium oxide nanoparticles as T1 magnetic resonance imaging (MRI)-cell labeling (CL) dual agents. RSC Adv 2:10907–10915
Fu J, Wang M, Zhang C, Xu Q, Huang X, Tang X (2012) Controlled fabrication of noble metal nanoparticles loaded on the surfaces of cyclotriphosphazene-containing polymer nanotubes. J Mater Sci 47:1985–1991
Li Z, Wang G, Ren W, Zhang A, An L, Tian Y (2016) Cyclotriphosphazene-containing polymeric nanotubes: Synthesis, properties, and formation mechanism. J Mater Sci 51:4096–4103
Lock LL, LaComb M, Schwarz K, Cheetham AG, Lin Y, Zhang P (2013) Self-assembly of natural and synthetic drug amphiphiles into discrete supramolecular nanostructures. Farad Discuss 166:285–301
Funding
Financial support from the National Science Foundation of Zhejiang Province (LHDMZ22H300003) is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mehmood, S., Yu, H., Wang, L. et al. Studies on the Synthesis and Drug Release Behavior of Cross-Linked Poly(cyclotriphosphazene-co-fluorescein) Microspheres. J Polym Environ 30, 5119–5129 (2022). https://doi.org/10.1007/s10924-022-02472-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-022-02472-8