Abstract
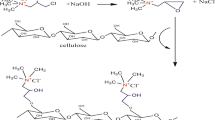
Microcrystalline cellulose (MCC) was modified with 1, 2, 3, 4-butanetetracarboxylic acid (BTCA) to obtain the adsorbent material named treated microcrystalline cellulose (TMCC), which was characterized for point of zero charge (pHZPC), estimation of carboxyl group content and surface group moieties, surface morphology and textural properties. Application of TMCC for the removal of Hg(II) ions from aqueous solution was studied with respect to carboxyl group content, and process parameters, including adsorbent dose, initial solution pH, temperature, contact time, and Hg(II) ion concentration, to provide information about the adsorption mechanism. Isothermal adsorption data were analysed using a range of two and three parameter adsorption models, with the level of fit determined using nonlinear regression analysis. The maximum adsorption capacity under optimised conditions was determined using Langmuir analysis and found to be 1140 mg/g, and Freundlich analysis showed that adsorption of Hg(II) ions onto TMCC is favourable. The kinetic results using a range of models, showed that a pseudo-second order kinetic model was most appropriate for the data obtained, which indicates that the process involves chemisorption. The results obtained show TMCC to have a high affinity for Hg(II) ions from aqueous media, which suggests that these materials may have potential for application in water treatment systems.









Similar content being viewed by others
References
Hokkanen S, Bhatnagar A, Sillanpaa M (2016) A review on modification methods to cellulose-based adsorbents to improve adsorption capacity. Water Res 91:156–173
Gusain D, Srivastava V, Sharma YC (2014) Kinetic and thermodynamic studies on the removal of Cu(II) ions from aqueous solutions by adsorption on modified sand. J Ind Eng Chem 20(3):841–847
Falayi T, Ntuli F (2014) Removal of heavy metals and neutralisation of acid mine drainage with un-activated attapulgite. J Ind Eng Chem 20(4):1285–1292
Janin A, Blais JF, Mercier G, Drogui P (2009) Selective recovery of Cr and Cu in leachatefrom chromate copper arsenate treated wood using chelating and acidic ion exchange resins. J Hazard Mater 169(1–3):1099–1105
Bratskaya SY, Pestov AV, Yatluk YG, Avramenko VA (2009) Heavy metals removal by flocculation/precipitation using N-(2-carboxyethyl) chitosans. Colloids Surf A 339(1–3):140–144
Tran TK, Leu HJ, Chiu KF, Lin CY (2017) Electrochemical treatment of heavy metal containing wastewater with the removal of COD and heavy metal ions. J Chin Chem Soc 64(5):493–502
Canet L, Ilpide M, Seta P (2002) Efficient facilitated transport of lead, cadmium, zinc, and silver across a flat-sheet-supported liquid membrane mediated by lasalocid A. Sep Sci Technol 37(8):1851–1860
Fenglian F, Qi W (2011) Removal of heavy metal ions from wastewaters: a review. J Environ Manage 92:407–418
Godwin PM, Pan Y, Xiao H, Afzal MT (2019) Progress in preparation and application of modified biochar for improving heavy metal ion removal from wastewater. J Bioresour Bioprod 4:31–42
Dong Z, Yuan W, Lib Y, Hua R, Zhao L (2019) Radiation synthesis of crown ether functionalized microcrystalline cellulose as bifunctional adsorbent: a preliminary investigation on its application for removal of ReO4- as analogue for TcO4-. Radiat Phys Chem 159:147–153
Wojnarovits L, Foldvary CM, Takacs E (2010) Radiation-induced grafting of cellulose for adsorption of hazardous water pollutants: a review. Radiat Phys Chem 79(8):848–862
Barsbay M, Güven O (2019) Surface modification of cellulose via conventional and controlled radiation induced grafting. Radiat Phys Chem 160:1–8
Boparai HK, Joseph M, O’Carroll DM (2011) Kinetics and thermodynamics of cadmium ion removal by adsorption onto nano zero valent iron particles. J Hazard Mater 186:458–465
Qi L, Teng F, Deng X, Zhang Y, Zhong X (2019) Experimental study on adsorption of Hg(II) with microwave-assisted alkali modified fly ash. Powder Technol 351:153–158
Fu Y, Jiang J, Chen Z, Ying S, Wang J, Hu J (2019) Rapid and selective removal of Hg(II) ions and high catalytic performance of the spent adsorbent based on functionalized mesoporous silica/ poly (m-aminothiophenol) nanocomposite. J Mol Liq 286:110746
Saman N, Kong H, Mohtar SS, Johari K, Mansor AF, Hassan O, Ali N, Mat H (2019) A comparative study on dynamic Hg(II) and MeHg(II) removal by functionalized agrowaste adsorbent: breakthrough analysis and adsorber design. Korean J Chem Eng 36:1069–1081
Zhang Y, Wang B, Cheng Q, Li X, Li Z (2020) Removal of toxic heavy metal ions (Pb, Cr, Cu, Ni, Zn Co, Hg, and Cd) from waste batteries or lithium cells using nanosized metal oxides: a review. J Nanosci Nanotechnol 20:7231–7254
Khalil M, Hashem A, Hebeish A (1990) Carboxymethylation of Maize Starch. Starch/Stärke 42:60–63
Kapoor A, Yang RT (1989) Correlation of equilibrium adsorption data of condensable vapours on porous adsorbents. Gas Sep Purif 3:187–192
Rangabhashiyam S, Anu N, Nandagopal M (2014) Relevance of isotherm models in biosorption of pollutants by agricultural by products. J Env Chem Eng 2(1):398–414
Kumar KV, Sivanesan S (2006) Pseudo second order kinetics and pseudo isotherms for malachite green onto activated carbon: comparison of linear and nonlinear regression methods. J Hazard Mater B 136:721–726
Ng JCY, Cheung WH, McKay G (2002) Equilibrium studies of the sorption of Cu(II) ions onto chitosan. J Colloid Interface Sci 255:64–74
Marquardt DW (1963) An algorithm for least-squares estimation of nonlinear parameters. J Soc Ind Appl Math 11:431–441
Boulinguiez B, Le Cloirec P, Wolbert D (2008) Revisiting the determination of Langmuir parameters application to tetrahydrothiophene adsorption onto activated carbon. Langmuir 24:6420–6424
Vijayaraghavan K, Padmesh TVN, Palanivelu K, Velan M (2006) Biosorption of nickel(II) ions onto Sargassum wightii: application of two-parameterand three-parameter isotherm models. J Hazard Mater 133:304–308
Ahsan HM, Zhang X, Li Y, Li B, Liu S (2019) Surface modification of microcrystalline cellulose: physicochemical characterization and applications in the stabilization of pickering emulsions. Int J Biol Macromol 132:1176–1184
Shi C, Tao F, Cui Y (2018) Evaluation of nitriloacetic acid modified cellulose film on adsorption of methylene blue. Int J Biol Macromol 114:400–407
Kong S, Huang X, Liand K, Song X (2019) Adsorption/desorption isotherms of CH4 and C2H6 on typical shale samples. Fuel 255:115632
Hamdaoui O (2006) Batch study of liquid-phase adsorption of methylene blue using cedar sawdust and crushed brick. J Hazard Mater 135:264–273
Ngah WWL, Teong R, Toh M (2012) Utilization of chitosan–zeolite composite in the removal of Cu(II) from aqueous solution: adsorption, desorption and fixed bed column studies. Chem Eng J 209:46–53
Martín-Lara M, Hernáinz F, Calero M, Blázquez G, Tenorio G (2009) Surface chemistry evaluation of some solid wastes from olive-oil industry used for lead removal from aqueous solutions. Biochem Eng J 44:151–159
Chen HY, Zhao A (2007) Removal of Cu(II) from aqueous solution by adsorption onto acid-activated palygorskite. J Hazard Mater 149:346–354
Khalil AA, Sokker HH, Al-Anwar A, Abd El-Zaher A, Hashem A (2009) Preparation, characterization and utilization of amidoximated poly(AN/MAA)-grafted alhagi residues for the removal of Zn(II) ions from aqueous solution. Adsorp Sci Technol 27:363–382
Langmuir I (1916) The constitution and fundamental properties of solids and liquids. Part I. Solids. J Am Chem Soc 38:2221–2295
Freundlich H (1907) Über die adsorption in lösungen Zeitschrift für physikalische. Chemie 57:385–470
Temkin M (1940) Kinetics of ammonia synthesis on promoted iron catalysts Acta Physiochim. URSS 12:327–356
Dubinin MM (1965) Modern state of the theory of volume filling of micropore adsorbents during adsorption of gases and steams on carbon adsorbents. Zh Fiz Khim 39:1305–1317
Redlich O, Peterson DL (1959) A useful adsorption isotherm. J Phys Chem 63:1024–1026
Toth J (1971) State equations of the solid gas interface layer. Acta Chem Acad Hung 69:311–317
Sips R (1948) Combined form of Langmuir and Freundlich equations. J Chem Phys 16:490–495
Foo KY, Hameed BH (2010) Insights into the modeling ofadsorption isotherm systems. Chem Eng J 156:2–10
Lagergren SK (1898) About the theory of so-called adsorption of soluble substances. Sven Vetensk Handingarl 24:1–39
Ho Y-S, McKay G (1999) Pseudo-second order model for sorption processes. Process Biochem 34:451–465
Weber WJ, Morris JC (1963) Kinetics of adsorption on carbon from solution. J Sanit Eng Div 89:31–60
Marrakchi F, Ahmed M, Khanday W, Asif M, Hameed B (2017) Mesoporous-activated carbon prepared from chitosan flakes via single-step sodium hydroxide activation for the adsorption of methylene blue. Int J Biol Macromol 98:233–239
Kołodyńska D, Hałas P, Franus M, Hubicki Z (2017) Zeolite properties improvement by chitosan modification—sorption studies. J Ind Eng Chem 52:187–196
Tutem E, Apak R, Unal CF (1998) Adsorptive removal of chloro phenols from water by bituminous shale. Water Res 32:2315–2324
Chien SH, Clayton WR (1980) Application of Elovich equation to the kinetics of phosphate release and sorption in soils. Soil Sci Soc Am J 44:265–268
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hashem, A., Mohamed, L.A., Fletcher, A.J. et al. Carboxylated Cellulose for Adsorption of Hg(II) Ions from Contaminated Water: Isotherms and Kinetics. J Polym Environ 29, 3040–3053 (2021). https://doi.org/10.1007/s10924-021-02075-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-021-02075-9