Abstract
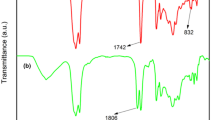
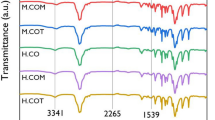
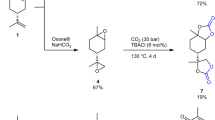
A three-step synthetic route was proposed and tested to obtain a chain-end functional di-sorbitan oleate monomer: First, 1,18-octadec-9-enedioic acid compound was produced by self-metathesis reaction of an oleic acid; then, the 1,18-octadec-9-enoyl dichloride compound was yielded by chlorination of the di-acid with thionyl chloride, and finally, the 1,18-di-sorbitan oleate monomer was yielded by esterification of the dichloride with 1,4-sorbitan. The di-sorbitan oleate monomer was purified and then characterized by FTIR, 1H-NMR, DSC and TGA to establish its structure and properties. A bio-based polyurethane (PU) was synthesized by reacting the obtained 1,18-di-sorbitan oleate monomer and MDI. Rheological analysis showed that a curing reaction occurs as a significant increase of the storage modulus (G’) and the complex viscosity (η*) at 100 °C. The obtained bio-based PU was characterized by FTIR, TGA and DMA, confirming that 1,18-di-sorbitan oleate is a feasible monomer for synthesizing polyurethanes.













Similar content being viewed by others
References
Mülhaupt R (2013) Macromol Chem Phys 214:159
Budzianowski WM (2017) Renew Sustain Energy Rev 70:793
Sheldon RA (2016) J Mol Catal A: Chem 422:3
Wang J et al (2017) Waste Manage 65:11
John G et al (2019) Prog Polym Sci 92:158
Zhang C et al (2017) Prog Polym Sci 71:91
Gandini A, Lacerda TM (2015) Prog Polym Sci 48:1
Salimon J, Salih N, Yousif E (2012) Arab J Chem 5(2):135
Yabushita M, Kobayashi H, Fukuoka A (2014) Appl Catal B 145:1
Rose M, Palkovits R (2012) Chemsuschem 5(1):167
Rusu OA et al (2015) Appl Catal B 176–177:139
Cao D et al (2016) Appl Catal A 528:59
Ginés-Molina MJ et al (2017) Appl Catal A 537:66
Cubo A et al (2017) Appl Catal A 531:151
Zhang Y et al (2018) Mol Catal 458:19
Dussenne C et al (2019) Mol Catal 463:61
Yuan D et al (2019) Appl Catal B 240:182
Gustini L et al (2015) Eur Polymer J 67:459
Bersot JC et al (2011) Macromol Chem Phys 212(19):2114
Brack, H., et al. (2011) US 2011/0077377A1 SABIC Innov Plast IP BV.
Jeong G-T et al (2007) Appl Biochem Biotechnol 137(1):935
Ng SH et al (2013) J Nanobiotechnol 11(1):27
Ciriminna R et al (2014) Sustain Chem Process 2(1):26
Hojabri L, Kong X, Narine S (2010) J Polym Sci A: Polym Chem 48(15):3302
More AS et al (2013) Eur Polymer J 49(4):823
Charlon M et al (2014) Eur Polymer J 61:197
del Río E et al (2011) Macromol Chem Phys 212(13):1392
Miao S et al (2012) Eur J Lipid Sci Technol 114(12):1345
Kalita H, Karak N (2014) J Appl Polym Sci 131(1):39579
Fourati Y et al (2017) Prog Org Coat 105:48
Mol J (1994) J Mol Catal 90(1–2):185
Mol J, Khosravi E, SzymanskaBuzar T (2002) Ring Open Metathesis Polym Relat Chem 56:377
Mol J (2004) Top Catal 27(1–4):97
Mol JC (2004) J Mol Catal A: Chem 213(1):39
Ngo H, Jones K, Foglia T (2006) J Am Oil Chem Soc 83(7):629
Ngo H, Foglia T (2007) J Am Oil Chem Soc 84(8):777
Zerkowski J, Solaiman D (2012) J Am Oil Chem Soc 89(7):1325
Kadyrov R et al (2012) Top Catal 55(7–10):538
Vyshnavi Y, Prasad RBN, Karuna MSL (2013) Ind Crops Prod 50:701
Aguilar-Castro C et al (2019) J Appl Polym Sci 136(8):47095
Yabushita , M. (2016) in a study on catalytic conversion of non-food biomass into chemicals: fusion of chemical sciences and engineering, Springer, Editor p.127
Yabushita M et al (2015) Bull Chem Soc Jpn 88(7):996
Petrović ZS et al (2010) Eur J Lipid Sci Technol 112(1):97
Miao S et al (2014) Acta Biomater 10:1692
Riyapan D, Saetung A, Saetung N (2019) J Polym Environ 27:1693
Dhaliwal GS, Anandan S, Chandrashekhara K et al (2019) J Polym Environ 27:1897
Bresolin D, Valério A, de Oliveira D et al (2018) J Polym Environ 26:2467
Zhuang JM, Steiner Paul R (1993) in Holzforschung – Int J Biol. Chem, Phys Technol Wood p, p 425
Yang PF, Han YD, Li TD (2011) Adv Mater Res 150–151:23
Lu M-Y et al (2018) Croat Chem Acta 91(3):299
Krämer RH et al (2010) Polym Degrad Stab 95(6):1115
Ketata N et al (2005) Polym Polym Compos 13(1):1
Acknowledgements
The authors wish to thank the National Council of Science and Technology of Mexico (CONACYT) for supporting this research work through the project CB-2015–01-257591.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Valencia-Bermudez, S., Hernández-López, S., Gutiérrez-Nava, M. et al. Chain-End Functional di-Sorbitan Oleate Monomer Obtained from Renewable Resources as Precursors for Bio-Based Polyurethanes. J Polym Environ 28, 1406–1419 (2020). https://doi.org/10.1007/s10924-020-01692-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-020-01692-0