Abstract
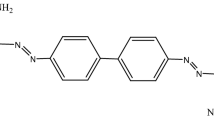
The present study concerns with exploring the possibility of using of tartaric acid pretreated sugarcane bagasse (SCB) for removing diazonium blue (DB) from aqueous solutions. The effect of different factors on the efficiency of the adsorbent for the DB dye removal was investigated, including initial dye concentration, contact time, SCB dosage and SCB particle size. Langmuir, Freundlich, Tempkin and D–R isothermal models have been employed to analyze the adsorption equilibrium data. It was found that the adsorption of the dye fits well with the D–R model. The adsorption kinetics was also done applying four kinetic models. The regression equation coefficients refer to fitting the data to the second-order kinetic equation for removal of the DB dye. It is probable that the rate limiting step is a chemical adsorption between the adsorbent and the dye. This chemisorption process is further confirmed from the energy value of 15.1 kJ mol−1 deduced from the D–R isotherm.

















Similar content being viewed by others
References
Crini G (2008) Kinetic and equilibrium studies on the removal of cationic dyes from aqueous solution by adsorption onto a cyclodextrin polymer. Dyes Pigm 77:415–426
Zhu L, Wang Y, He T, You L, Shen X (2016) Assessment of potential capability of water bamboo leaves on the adsorption removal efficiency of cationic dye from aqueous solutions. J Polym Environ 24:148–158
Wong YC, Ranjini KN, Wan-Nurdiyana WA (2014) Removal of Congo red and acid yellow 36 dyes using orange peel and rice husk as adsorbent. Orient J Chem 30:529–539
Mafra MR, Mafra LI, Zuim DR, Vasques EC, Feneira MA (2013) Adsorption of remazol brilliant blue on an orange peel adsorbent. Braz J Chem Eng 30:657–665
Ma C, Li Z, Zhao W, Xu Y, Gui Y, Xiu K, Huang (2004) Garlic peel as adsorbent for the removal of methylene blue from aqueous solution. Appl Mech Mater 694:367–371
Witek-Krowiak A (2003) Biosorption of malachite green from aqueous solutions by pine sawdust: equilibrium, kinetics and the effect of process parameters. Desalin Water Treat 51:3284–3294
Chieng HI, Lim LBL, Priyanthan N (2015) Enhancing adsorption capacity of toxic malachite green dye through chemically modified breadnut-peel: equilibrium, thermodynamics, kinetics and regeneration studies. Environ Technol 36:86–97
Adegoke KA, Bello O-S (2015) Dye sequestration using agricultural wastes as adsorbents. Water Resour Ind 12:8–24
Zhang P, Chi Chang C, Wang R, Zhang S (2014) Agricultural waste. Water Environ Res 86:1387–1415
Bharathi KS, Ramesh ST (2013) Removal of dyes using agricultural waste as low-cost adsorbents: a review. Appl Water Sci 3:773–790
Saad M, Tahir H, Al D (2017) Green synthesis of Ag–Cr–AC nanocomposites by Azadirachtaindica and its application for the simultaneous removal of binary mixture of dyes by ultrasonicated assisted adsorption process using response surface methodology. Ultrason Sonochem 38:197–213
Saad M, Tahir H, Al D (2017) Synthesis of carbon loaded γ-Fe2O3 nanocomposite and their applicability for the selective removal of binary mixture of dyes by ultrasonic adsorption based on response surface methodology. Ultrason Sonochem 36:393–408
Asfaram A, Ghaedi M, Yousefi F, Dastkhoon M (2016) Experimental design and modeling of ultrasound assisted simultaneous adsorption of cationic dyes onto ZnS: Mn-NPs-AC from binary mixture. Ultrason Sonochem 33:77–89
Johnson A (1989) In: Johnson A (ed) The theory of coloration of textile. Society of Dyers and Colourists, Huddersfield, p 477 (Chapter 7)
Green FG (1990) The Sigma-Aldrich Handbook of stains, dyes and indicators. Aldrich Chemical Company, Inc, Milwaukee
Said AA, Aly AAM, Abd El-Wahab MMM, Soliman SA, Abd El- Hafez AA, Helmy V, Goda MN (2012) Potential application of propionic acid modified sugarcane bagasse (SCB) for removal of basic and acid dyes from industrial wastewater. Res Environ 2:93–99
Srivastava R, Rupainwar DC (2011) A comparative evaluation for adsorption of dye on neem bark and mangs bark powder. Indian J Chem Technol 18:67–75
Hassanein T, Koumanova B (2010) Evaluation of adsorption potential of the agricultural waste wheat straw for basic yellow 21. J Univ Chem Technol Metall 45:407–414
Bulut Y, Aydin H (2006) A kinetics and thermodynamics study of methylene blue adsorption on wheat shells. Desalination 194: 259–267
Said AA, Aly AAM, Abd El-Wahab MM, Soliman SA, Abd El- Hafez AA, Helmy V, Goda MN (2013). Application of modified sugarcane bagasse (SCB) as a biosorbent for relative dyes removal from industrial wastewater. J Water Res Prot 5:10–17
Yakout SM, Elsherif E (2010) Batch kinetics, isotherm and thermodynamic studies of adsorption of strontium from aqueous solutions onto low cost rice-straw based carbons. Carbon-Sci Technol 1:144–153
Sultan M, Tahir H, Ahmed K, Jahanzeb Q (2011) The kinetics study of the reduction of Fast Green dye with cetylpyridinum chloride as cationic surfactant. Front Chem China 6(2):105–112
Saad M, Tahir H, Khan J, Hameed U, Saud A (2017) Synthesis of polyaniline nanoparticles and their application for the removal of crystal violet dye by ultrasonicated adsorption process based on response surface methodology. Ultrason Sonochem 34:600–608
Tahir H, Sultan M, Akhtar N, Hameed U, Abid T (2016) Application of natural and modified sugar cane bagasse for the removal of dye from aqueous solution. J Saudi Chem Soc 20:S115–S121
Alves MJ, Cavalcanti CV, de Resende MM, Cardoso VL, Reis MH (2016) Biodiesel dry purification with sugarcane bagasse. Ind Crops Prod 89:119–127
Acknowledgements
The authors would like to thank the financial support from the Science and Technology Development Fund, STDF, Ministry of Higher Education, Egypt.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Said, A.EA.A., Aly, A.A.M., Goda, M.N. et al. Modified Sugarcane Bagasse with Tartaric Acid for Removal of Diazonium Blue from Aqueous Solutions. J Polym Environ 26, 2424–2433 (2018). https://doi.org/10.1007/s10924-017-1136-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-017-1136-9