Abstract
Gait and dynamic balance are two main goals in neurorehabilitation that mHealth systems could address. To analyze the impact of using mHealth systems on gait and dynamic balance outcomes in subjects with neurological disorders. Randomized controlled trials (RCT) published in PubMed, Web of Science, Scopus, and PEDro databases were searched up to April 2023. Studies including adults with neurological disorders, analyzing the effectiveness of mHealth systems on gait and dynamic balance compared with conventional therapy and/or not intervention, were included. The PEDro scale and the Cochrane Collaboration’s 2.0 tool were used for the methodological quality and risk of bias assessment. The Review Manager 5.4 software was used to obtain meta-analyses. 13 RCT were included in the systematic review and 11 in the meta-analyses, involving 528 subjects. A total of 21 mobile applications were identified for gait and balance training, and to enhance physical activity behaviors. There were significant differences in gait parameters, speed by 0.10 s/m (95% confidence interval (CI)=0.07,0.13;p<0.001), cadence by 8.01 steps/min (95%CI=3.30,12.72;p<0.001), affected step length by 8.89 cm (95%CI=4.88,12.90;p<0.001), non-affected step length by 8.08 cm (5%CI=2.64,13.51;p=0.004), and in dynamic balance, Timed Up and Go by -7.15 s (95%CI=-9.30,-4.99;p<0.001), and mobility subscale of Posture Assessment Scale for Stroke by 1.71 points (95%CI=1.38,2.04;p<0.001). Our findings suggested the use of mHealth systems for improving gait in subjects with neurological disorders, but controversial results on dynamics balance recovery were obtained. However, the quality of evidence is insufficient to strongly recommend them, so further research is needed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Motor impairments represent a leading cause for the limitation of functional activities and disability in subjects with neurological disorders [1,2,3,4]. In particular, they require gait and balance for community ambulation and autonomy, which are indirectly related to participation, one domain in the International Classification of Functioning, Disability and Health (ICF) model [5, 6]. Moreover, a loss of postural control during gait increases the likelihood of falls [7, 8]. Therefore, a key goal of neurorehabilitation is to restore mobility through gait [9, 10].
In that way, mHealth, mobile device-based healthcare [11], involves the practice of medicine and public health based on mobile devices to improve and promote health status [12]. It is considered a domain of telerehabilitation when it applies to rehabilitation programs [13]. The interest in mHealth has particularly boomed because of the growth of smartphone access and the use of mobile applications, commonly called “apps” [14]. In the neurorehabilitation field, there are apps to monitor vital signs [15], detect neurological symptomatology [16,17,18], track neurodegenerative diseases [19,20,21], treat motor and cognitive impairments [22,23,24,25], among others. In that sense, Dorsey et al. [26] reviewed the global proliferation and current state of telerehabilitation in neurological disorders. They highlighted the potential to increase access to neurological care and education by telerehabilitation. Likewise, Dobkin [27] suggested mHealth enables therapists to remotely monitor adherence and to obtain continuous outcomes measurements over time, allowing the assessment of goal attainment in the daily routine. Consequently, the use of these technologies facilitates the self-management of the rehabilitation process and becomes the subject more active, increasing participation. Because of this background, mHealth could complement multimodal programs in neurorehabilitation, making it more effective, including different sensorial feedback, motor experiences, and increasing the subject’s motivation [28].
Concerning the current scientific literature, some reviews are analyzing the potential use of apps in subjects with neurological disorders [13, 29,30,31]. Nevertheless, authors mainly analyzed commercial apps available in repositories, whose scientific evidence was slightly based on randomized clinical trials (RCT). To our knowledge, there is not a review and meta-analysis examining the use of mHealth systems in neurorehabilitation, specifically focused on their impact on gait and balance outcomes. Consequently, the main aim of this study was to analyze the impact of the use of mHealth systems on gait and dynamic balance outcomes in subjects with neurological disorders, performing a systematic review and meta-analysis.
Methods
Search strategy
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines for systematic reviews of randomized controlled trials [32] was followed to report the present systematic review and meta-analysis (Online resource 1). The search was performed up to April 2023 in the following databases: PubMed, Web of Science, Scopus, and Physiotherapy Evidence Database (PEDro). It was adapted for the rest of the databases from an initial search of the PubMed database (Online resource 2). No filters were used in terms of language or publication date.
Eligibility criteria
The inclusion criteria were defined according to the PICOS framework [33] (Population, Intervention, Comparison, Outcomes, Studies): i) RCT including adults (over 18 years old) with neurological disorders, ii) comparing mHealth systems (smartphones or tablets) with a control group, iii) mHealth systems used during gait and balance training, apps based on physical activity (PA) programs in real-world settings, and mobile apps providing feedback to enhance self-patient management and participation to rehabilitation programs, iv) Control group was based on conventional therapy (physical and/or occupational therapies, such as specific gait and balance training, home-based PA programs and active behavior recommendations) and/or not intervention, v) gait and dynamic balance as outcomes.
The exclusion criteria were: i) Systems based on videoconferencing platforms (e.g. Skype, Zoom, etc.), virtual reality glasses, or websites, ii) Studies related to cost-effectiveness and usability of mHealth systems.
Study selection
Two authors (M.M.L and J.A.M.M.) retrieved and screened the studies by the titles and abstracts independently, removing duplicate articles and identifying potentially relevant articles according to the pre-established criteria. Subsequently, full texts of the retrieved records were downloaded and analyzed for final inclusion in the systematic review. An additional reviewer (D.L.A.) was considered for consensus when needed. Some information was extracted to detail the studies characteristics: authors, year of publication, country, characteristics of the participants (number of participants in both groups, mean age, neurological disorder), and the intervention characteristics (types, session length and frequency, total duration, outcomes measured, measurement instruments, and results). In addition, each mHealth system employed as an intervention was identified, specifying its purpose and main features.
Quality assessment
The PEDro scale [34] was used to evaluate the methodological quality of the studies. A study scored as 6 or more is considered having good methodological quality (6-8: good; 9-10: excellent) and scored as 5 or less is considered being of acceptable or poor quality (4-5: acceptable; <4: poor) [35]. The Cochrane Risk of Bias (RoB) Collaboration’s 2.0 tool [36] was used for assessing the risk of bias. This evaluation covers five domains of bias: (1) bias arising from the randomization process, (2) bias due to deviations from intended interventions, (3) bias due to missing outcome data, (4) bias in measurement of the outcome, and (5) bias in selection of the reported result. The RoB of each domain was categorized as: “low risk”, “some concerns”, and “high risk”. Also, an overall RoB assessment of the RCT was performed according to the recommendations in the guidance document. Those assessments were performed by two authors (M.M.L and J.A.M.M.) independently. When there was a difference in the scores between the authors, the final decision was discussed with a third author (D.L.A.).
Statistical analysis
Several meta-analyses were performed with The Review Manager 5.4 software to summarize the comparisons of the use of mHealth systems (experimental group) versus a control group. The studies were classified based on the measuring instrument to assess gait or dynamic balance. The effect measure was the mean difference (MD), reported along with their 95% confidence intervals (CI). The instructions of the Cochrane Handbook for Systematic Reviews of Interventions for obtaining standard deviation from confidence intervals and change-from-baseline standard deviation were employed when needed. After checking for homogeneity with the chi-square test and the I2 statistic (0-40% might not be important; 30-60% may represent moderate heterogeneity; 50-90% may represent substantial heterogeneity; and 75-100% considerable heterogeneity) [37], a fixed-effect model was used when no heterogeneity was detected, and a random model was used otherwise. The significance level was set at alpha=0.05, and the results were presented in forest plots.
Results
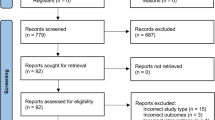
A total of 594 articles were retrieved during the initial search and 13 RCT were included in the systematic review and 11 in the meta-analysis (Fig. 1). The studies by Asano et al. [38] and Hankinson et al. [39] were excluded because it did not provide the necessary data for the meta-analysis. In that way, 528 subjects participated in the included studies.
Studies characteristics
The average age was 60.4, although three documents did not provide information [39,40,41]. All studies included both genders and the most common disease was stroke, studied in eight articles [38, 42,43,44,45,46,47] (Table 1).
Regarding the mHealth systems, they can be classified as those specifically focused on gait training, balance training or those based on home-based PA programs. First, gait training was performed by four different apps: i) ZyMiMetronome App [47] and GotRhythm App [39], two metronome apps that provided a rhythmic auditory stimulation during gait training; ii) Virtual Active App [44], used for speed-interactive treadmill training providing visual feedback through filmed world-famous landscapes; and iii) Audio-biofeedback (ABF)-gait and Freezing of Gait (FOG)-cue Apps [41], part of the CuPiD system, provided biofeedback by verbal instructions and visual information, respectively, during gait recovery. Second, for balance training, a group of apps called CSMi [45] was used, included in the SPVFTCT (smartphone-based visual feedback trunk control training) system, designed for trunk control training. It allowed real-time feedback through the monitoring of weight shifts during the execution of the proposed goal task. Similarly, the Compass Inclinometer App [42] was used for providing patients visual feedback while performing a home-based sitting balance training program. Another mHealth system used for balance training was the Farmalarm App [43], which guided home-based core stability exercise (CSE) through description, photo, and video of each exercise, and recorded its performance. Finally, we found apps providing exercise programs to enhance PA. They were not specifically designed for training gait and balance, but the impact of these apps on these outcomes was assessed. The apps used were Wellpepper App [48], Fitlab Training App [46], Lose it! [49], iPro Habit Tracker Apps [49], Memories App [49], Pt Pal App [40], Patient Information App (PIA) [50], and ad-hoc app [38]. They provided home-based PA programs including videos and prescription information, allowing the monitoring (behaviors, symptoms, or goal achievement), the guidance of the PA performance (visual and/or auditory), and the communication with their physical therapist. More specifications about the mHealth systems employed are shown in Table 2.
Regarding the control interventions, conventional physical and occupational therapies were included, such as specific gait and balance training, home-based PA programs, and active behavior recommendations. None included study compared the mHealth group with no intervention or waitlist.
Concerning the measuring instruments, several motion analysis systems were used (GAITRite platform [47], OptoGait system [44], and ProtoKinetics platform [41]) for gait spatiotemporal analysis. Furthermore, the 10-meter Walk Test (10MWT) [40, 46] was also used for the gait speed analysis. For gait and dynamic balance analysis, the Timed Up and Go (TUG) test [40, 45,46,47], the Four Squared Step Test (FSST) [41, 47], the 6-minute Walk Test (6MWT) [40, 46, 48,49,50], the 2-minute Walk Test (2MWT) [41, 50], and the mobility subscale of the Postural Assessment Scale for Stroke (PASS) [42, 43] were used.
Quality assessment
The overall PEDro score was 6.23 (range 3-8), implying a moderate quality of the articles included in this review. Ten studies [38, 40,41,42,43,44,45, 48,49,50] obtained a good methodological quality, two were acceptable [46, 47], and one was poor [39] (Online resource 3). Concerning the assessment of the RoB by domain, 7 studies had low RoB and the rest had some concerns for the allocation domain. For the second domain (bias due to deviations from intended interventions), 9 studies had low RoB while 4 studies had some concerns. In case of third (missing outcome data) and fourth (measurement of the outcomes) domains, 11 and 10 studies had low RoB, respectively. For the last domain, in the selection of the reported results, 11 studies had low RoB and only one study had some concerns (Online resource 4). Regarding the overall judgement, 5 studies had a low RoB, 5 studies had some concerns, and 3 studies had a high RoB, as shown in Online resource 4.
Results on gait and dynamic balance
The mean, standard deviation and sample sizes of the different subgroups and measures, along with the MD (95%CI), are shown in Figs. 2 and 3. No statistical heterogeneity was observed in seven of the ten outcomes studied. However, stride length and FSST presented moderate heterogeneity (I2=49% and I2=47%, respectively), while PASS (mobility) presented substantial heterogeneity (I2=75%). We found significant differences in temporal gait parameters, speed (MD=0.10;95%CI=0.07,0.13; p<0.001) (Fig. 2A) and cadence (MD=8.01;95%CI=3.30,12.72; p<0.001) (Fig. 2B). Regarding spatial gait parameters, the stride length results were no significant (MD=0.08;95%CI=-0.00,0.16; p=0.06) (Fig. 2C). Conversely, there were significant differences in affected step length outcome (MD=8.89;95%CI=4.88,12.90; p<0.001) (Fig. 2D) and non-affected step length (MD=8.08;95%CI=2.64,13.51; p=0.004) (Fig. 2E). Concerning the dynamic balance, significant differences were observed in the TUG test (MD=-7.15;95%CI=-9.30,-4.99; p<0.001) (Fig. 3A), and PASS (mobility) (MD=1.71;95%CI=1.38,2.04; p<0.001) (Fig. 3B). The FSST (MD=-1.90;95%CI=-3.82,0.03; p>0.05) (Fig. 3C), 6MWT (MD=11.74;95%CI=-16.40,39.89; p=0.41) (Fig. 3D), and 2MWT (MD=5.13;95%CI=-10.55,20.81; p=0.52) (Fig. 3E) results were no significant. Therefore, the results of the present study suggest that the use of mHealth systems was slightly more effective than the conventional rehabilitation of training and enhancing gait and balance outcomes according to motion analysis systems, the TUG test, and the PASS (mobility).
Forest plots of gait spatiotemporal parameters. (A) Mean difference (95% CI) of effect of mHealth vs. conventional therapy on speed; (B) Mean difference (95% CI) of effect of mHealth vs. conventional therapy on cadence; (C) Mean difference (95% CI) of effect of mHealth vs. conventional therapy on stride length; (D) Mean difference (95% CI) of effect of mHealth vs. conventional therapy on affected step length; (E) Mean difference (95% CI) of effect of mHealth vs. conventional therapy on non-affected step length; CI: Confidence interval; IV: Inverse variance; SD: Standard deviation
Forest plots of dynamic balance. (A) Mean difference (95% CI) of effect of mHealth vs. conventional therapy on TUG; (B) Mean difference (95% CI) of effect of mHealth vs. conventional therapy on PASS (mobility); (C) Mean difference (95% CI) of effect of mHealth vs. conventional therapy on FSST; (D) Mean difference (95% CI) of effect of mHealth vs. conventional therapy on 6MWT; (E) Mean difference (95% CI) of effect of mHealth vs. conventional therapy on 2MWT; CI: Confidence interval; IV: Inverse variance; SD: Standard deviation
Discussion
The present systematic review and meta-analysis were performed to analyze the impact of the use of mHealth systems on gait and dynamic balance outcomes in subjects with neurological disorders. To our knowledge, this is the first systematic review and meta-analysis analyzing the use of mHealth systems for improving gait and balance in neurorehabilitation, providing the first evidence-based findings supporting its clinical application. As a result, 13 studies were reviewed and 11 were examined in this meta-analysis. Therefore, and according to our results, there was some evidence of the use of mHealth systems which can impact positively on gait and balance outcomes in subjects with neurological disorders.
There are some reviews [13, 29,30,31] of both literature and apps markets conducted to analyze the commercial mobile apps in the neurorehabilitation field. They stated that despite the wide variety of available apps with potential use and design for subjects with neurological disorders, the scientific evidence from RCT is also limited to generalize their efficacy and merge their recommendation. In addition, they recommended knowing the characteristics and therapeutic needs of this population to develop well-founded and feasibility apps. Accordingly, a recent review [51] also recommended the need to design new and mixed teleneurorehabilitation protocols, which will be focused on measuring quantitative and qualitative measures simultaneously. Another widespread conclusion in mHealth research is the content validation, for which there are several evaluation tools [52].
Gait recovery is the key goal in neurorehabilitation [53], and new modalities of intervention are emerging. In that way, one of these modalities is the use of the mHealth systems for improving spatiotemporal gait parameters, and those parameters are reliable measurements to quantify gait recovery [54]. There are different systems for recording, assessing, and monitoring motor impairments in daily life settings for the population with neurological disorders. In our meta-analysis, the findings on walking speed, cadence, and affected and non-affected step length showed significant improvements, so it can be considered a promising and effective training method to improve those parameters. Nevertheless, the results should be taken with caution because of the differences in assessment systems and tests used, studied population and the lightly studies included.
Gait impairments are closely related to decreased motor trunk control and postural balance. The involvement of similar brain centers and neural pathways could explain this relationship for integrating neuromuscular and sensory signals [55]. Therefore, neurorehabilitation should aim to improve both functions to facilitate daily life activities recovery for independent and social participation [56], as well as to decrease the risk of falls in population with neurological disorders [41]. In this sense, our results showed controversial findings depending on the measuring instrument. It is important to highlight that the study using the CSMi apps [45], is the only intervention designed for balance training, measuring the dynamic balance with the TUG test, but not with FSST and 6MWT. It might influence our results, getting significant results for the TUG test, but not for FSST, 6MWT and 2MWT.
The improvements in gait and balance using mHealth could be enhanced by several strategies, such as auditory feedback, visual feedback, or incentive-based approaches. Concerning the ZyMi Metronome App [47] and GotRhythm App [39], they provide gait training through rhythmic auditory stimulation for exciting motor neurons in the brainstem and the spinal level, promoting motor learning and changes in neuroplasticity through audition [57]. In that way, there is wide evidence of the rhythmic auditory stimulation effects in recovering spatiotemporal gait parameters and dynamic balance in subjects with neurological disorders [58,59,60]. Furthermore, ABF-gait App [41] provided auditory feedback using verbal corrections and positively reinforce during gait performance. Moreover, there are smartphone apps used in the included studies which provide visual feedback, such as Virtual Active App [44], FOG-cue App [41], CSMi Apps [45], Compass Inclinometer App [42], or Farmalarm App [43], during the intervention to guide motor performance. According to Noh et al. [56], it may be more effective than conventional training for gait and balance recovery. Finally, there are incentive-based mHealth apps, such as Wellpepper App [48], Fitlab Training App [46], iPro Habit Tracker App [49], Pt Pal App [40], Patient App [38] or PIA [50], which provide home-based PA programs, PA information and recommendations with activity goal attainments and rewards, increasing the subjects’s motivation and the adherence to PA [46].
Concerning the baseline characteristics of the participants, eight studies [38, 42,43,44,45,46,47] included subjects with stroke, in subacute or chronic phases. Two studies [41, 48] involved subjects with Parkinson’s disease, other two [40, 49] included a sample of subjects with several neurological disorders, and one study included subjects suffering multiple sclerosis [50]. Therefore, the functional characteristics of the included participants were heterogeneous. In addition, the sample size was relatively limited in most included studies with only 20-30 participants, making it difficult to establish adequate and solid conclusions. It is important to consider that these types of studies are usually performed in clinical or home settings, without controlling the environmental influence and involving patients receiving treatment in an institution. Therefore, it is necessary to perform multi-centered studies with no convenience samples, so it may generate selection bias [61].
Aiming to complete our insight about the analyzed topic, the minimum clinically important differences (MCID) were considered evaluating the clinical relevance of our results. According to the speed gait parameter, it was estimated that the MCID in subjects with stroke is 0.16 m/s [62] for subacute phases and an overall value of 0.13 m/s [63] was determined. For Parkinson’s disease, it was established as the range of clinically important difference an enhanced speed gait from 0.05 to 0.22 m/s, considering 0.14 m/s as moderate change [64]. In any case, despite the statically significant difference in gait speed between studied groups, it cannot be considered a clinically relevant change. Regarding dynamic balance, the MCID of the TUG test is stated as -3.8 to 2.6 seconds for subjects with stroke [65]. In this instance, our results of the TUG test reached that clinical relevance. Although the overall results of our meta-analyses were significant for cadence, step length and PASS (mobility) in subjects with neurological diseases, the information about the MCID was not found. Therefore, we recommend the scientific community provide future studies results in this way.
Finally, regarding the role of the neurorehabilitation professionals, they have an active role on guiding patients on the effective use of mHealth tools and ensuring that the interventions are safe and well-conducted [66]. By doing so, they can help patients managing their conditions more effectively and improving their overall health outcomes. They also assist patients, families, and caregivers to have clear criteria and indicators to use the optimal mHealth tools for their specific requirements [30]. Definitively, professionals need to know the validation data and scientific evidence of each app, as well as their approval by the corresponding control agencies [67], in order to individualize the clinical recommendations to the needs of the patients with neurological disorders.
Study limitations
Although this review and meta-analysis provide an evidence-based overview of the use of mHealth in subjects with neurological disorders, some limitations should be remarked. Our results should be taken with caution because of the heterogeneity of neurological disorders and the variety of assessment tools used. Likewise, several intervention protocols were used in experimental and control groups. These facts could influence the comprehension and interpretation of findings. Thus, protocols standardization is needed for future studies. Another common limitation, along with the studies included, was the lack of assessor blinding for group allocation, resulting in a high risk of bias in this domain. As well as stated previously, the sample size involved in the studies was limited, making the generalization of the obtained results difficult. Finally, it is necessary to perform trials with long-term follow-up to assess whether the mHealth effects are maintained in neurorehabilitation.
Nevertheless, despite of those limitations, this systematic review and meta-analysis provides the first evidence-based findings about the use of mHealth systems for gait and balance recovery in neurorehabilitation. Therefore, this manuscript could be used as the base of future clinical studies and discover the necessity of further research that will allow the stratified analysis of the results.
Conclusions
In conclusion, our study showed some evidence that mHealth systems can be a useful tool for subjects with neurological disorders to improve gait, and controversial results were found on dynamic balance recovery. In that way, the use of smartphones and tablets for performing clinical or home-based rehabilitation is still in its first stages, and the quality of evidence is not enough to merge the effectiveness and strongly recommend the use of these systems in neurorehabilitation. Thus, it is needed to conduct clinical trials with higher methodological quality, involving a larger sample size, homogenous studied groups, standardized protocols, and outcome measures to recommend mHealth as an alternative or reliable complement to clinical neurorehabilitation.
References
M.A. Dimyan, L.G. Cohen, Neuroplasticity in the context of motor rehabilitation after stroke, Nat Rev Neurol. 7 (2011) 76–85. https://doi.org/10.1038/nrneurol.2010.200.
N.E. Mayo, S. Wood-Dauphinee, S. Ahmed, C. Gordon, J. Higgins, S. McEwen, N. Salbach, Disablement following stroke, Disabil Rehabil. 21 (1999) 258–268. https://doi.org/10.1080/096382899297684.
S. Sveinbjornsdottir, The clinical symptoms of Parkinson’s disease, J Neurochem. 139 (2016) 318–324. https://doi.org/10.1111/jnc.13691.
H.K. Graham, P. Rosenbaum, N. Paneth, B. Dan, J.P. Lin, Di.L. Damiano, J.G. Becher, D. Gaebler-Spira, A. Colver, Di.S. Reddihough, K.E. Crompton, R.L. Lieber, Cerebral palsy, Nat Rev Dis Primers. 2 (2016). https://doi.org/10.1038/nrdp.2015.82.
C.C. Oliveira, A. Lee, C.L. Granger, K.J. Miller, L.B. Irving, L. Denehy, Postural Control and Fear of Falling Assessment in People With Chronic Obstructive Pulmonary Disease: A Systematic Review of Instruments, International Classification of Functioning, Disability and Health Linkage, and Measurement Properties, Arch Phys Med Rehabil. 94 (2013) 1784-1799.e7. https://doi.org/10.1016/j.apmr.2013.04.012.
WHO, World Health Organization, Geneva, World Report on Child Injury Prevention. (2001).
A. Mirelman, P. Bonato, R. Camicioli, T.D. Ellis, N. Giladi, J.L. Hamilton, C.J. Hass, J.M. Hausdorff, E. Pelosin, Q.J. Almeida, Gait impairments in Parkinson’s disease, Lancet Neurol. 18 (2019) 697–708. https://doi.org/10.1016/S1474-4422(19)30044-4.
L. Comber, R. Galvin, S. Coote, Gait deficits in people with multiple sclerosis: A systematic review and meta-analysis, Gait Posture. 51 (2017) 25–35. https://doi.org/10.1016/j.gaitpost.2016.09.026.
K.H. Mauritz, Gait training in hemiplegia, European Journal of Neurology, Supplement. 9 (2002) 23–29. https://doi.org/10.1046/j.1468-1331.2002.0090s1023.x.
C.L. Tomlinson, S. Patel, C. Meek, C.P. Herd, C.E. Clarke, R. Stowe, L. Shah, C. Sackley, K.H.O. Deane, K. Wheatley, N. Ives, Physiotherapy intervention in Parkinson’s disease: Systematic review and meta-analysis, BMJ (Online). 345 (2012) 1–14. https://doi.org/10.1136/bmj.e5004.
E.M. Giesbrecht, W.C. Miller, Effect of an mHealth Wheelchair Skills Training Program for Older Adults: A Feasibility Randomized Controlled Trial, Arch Phys Med Rehabil. 100 (2019) 2159–2166. https://doi.org/10.1016/j.apmr.2019.06.010.
S.R. Steinhubl, E.D. Muse, E.J. Topol, The emerging field of mobile health, Sci Transl Med. 7 (2015) 1–7. https://doi.org/10.1126/scitranslmed.aaa3487.
J.A. Moral-Munoz, B. Esteban-Moreno, E. Herrera-viedma, M.J. Cobo, I.J. Pérez, Smartphone Applications to Perform Body Balance Assessment: a Standardized Review, J Med Syst. 42 (2018). https://doi.org/10.1007/s10916-018-0970-1.
F.H. McKay, C. Cheng, A. Wright, J. Shill, H. Stephens, M. Uccellini, Evaluating mobile phone applications for health behaviour change: A systematic review, J Telemed Telecare. 24 (2018) 22–30. https://doi.org/10.1177/1357633X16673538.
F.S. Sarfo, F. Treiber, M. Gebregziabher, S. Adamu, M. Nichols, A. Singh, V. Obese, O. Sarfo-Kantanka, A. Sakyi, N. Adu-Darko, R. Tagge, M. Agyei-Frimpong, N. Kwarteng, E. Badu, N. Mensah, M. Ampofo, C. Jenkins, B. Ovbiagele, Phone-based intervention for blood pressure control among Ghanaian stroke survivors: A pilot randomized controlled trial, International Journal of Stroke. 14 (2019) 630–638. https://doi.org/10.1177/1747493018816423.
B.Y. Andrew, C.M. Stack, J.P. Yang, J.A. Dodds, mStroke: “Mobile Stroke”—Improving Acute Stroke Care with Smartphone Technology, Journal of Stroke and Cerebrovascular Diseases. 26 (2017) 1449–1456. https://doi.org/10.1016/j.jstrokecerebrovasdis.2017.03.016.
J. Cancela, S.V. Mascato, D. Gatsios, G. Rigas, A. Marcante, G. Gentile, R. Biundo, M. Giglio, M. Chondrogiorgi, R. Vilzmann, S. Konitsiotis, A. Antonini, M.T. Arredondo, D.I. Fotiadis, Monitoring of motor and non-motor symptoms of Parkinson’s disease through a mHealth platform, Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, EMBS. 2016-Octob (2016) 663–666. https://doi.org/10.1109/EMBC.2016.7590789.
M. Banky, R.A. Clark, B.F. Mentiplay, J.H. Olver, M.B. Kahn, G. Williams, Toward Accurate Clinical Spasticity Assessment: Validation of Movement Speed and Joint Angle Assessments Using Smartphones and Camera Tracking, Arch Phys Med Rehabil. 100 (2019) 1482–1491. https://doi.org/10.1016/j.apmr.2018.11.026.
S. Zhan, A. Mohan, S. Tarolli, C. Schneider, Ruth B. Adams, Jamie L. Sharma, S. Elson, M. J. Spear, K. L. Glidden, A. M. Little, M.A. Terzis, A. Dorsey, E. R. Saria, Using Smartphones and Machine Learning to Quantify Parkinson Disease Severity: The Mobile Parkinson Disease Score, JAMA Neurol. 75 (2018) 876–880. https://doi.org/10.1001/jamaneurol.2018.0809.
R.A. Marrie, S. Leung, T. Tyry, G.R. Cutter, R. Fox, A. Salter, Use of eHealth and mHealth technology by persons with multiple sclerosis, Mult Scler Relat Disord. 27 (2019) 13–19. https://doi.org/10.1016/j.msard.2018.09.036.
J.P. Berry, D. James, S. Paganoni, K. Carlson, K. Burke, H. Weber, P. Staples, J. Salinas, J. Chan, J.R. Green, R. Connaghan, K. Barback, J. Onnela, Design and results of a smartphone-based digital phenotyping study to quantify ALS progression, Ann Clin Transl Neurol. 6 (2019) 873–881. https://doi.org/10.1002/acn3.770.
T. Neuendorf, D. Zschäbitz, N. Nitzsche, H. Schulz, Movement Therapy of the Upper Extremities with a Robotic Ball in Stroke Patients: Results of a Randomized Controlled Crossover Study, Aktuelle Neurologie. 45 (2017) 434–444. https://doi.org/10.1055/s-0043-122220.
J. Ku, T. Lim, Y. Han, Y.J. Kang, Mobile Game Induces Active Engagement on Neuromuscular Electrical Stimulation Training in Patients with Stroke, Cyberpsychol Behav Soc Netw. 21 (2018) 504–510. https://doi.org/10.1089/cyber.2018.0045.
A. Lauraitis, R. Maskeliunas, R. Damasevicius, D. Polap, M. Wozniak, A Smartphone Application for Automated Decision Support in Cognitive Task Based Evaluation of Central Nervous System Motor Disorders, IEEE J Biomed Health Inform. 23 (2019) 1865–1876. https://doi.org/10.1109/JBHI.2019.2891729.
S.D. van der Linden, M.M. Sitskoorn, G.J.M. Rutten, K. Gehring, Feasibility of the evidence-based cognitive telerehabilitation program Remind for patients with primary brain tumors, J Neurooncol. 137 (2018) 523–532. https://doi.org/10.1007/s11060-017-2738-8.
E.R. Dorsey, A.M. Glidden, M.R. Holloway, G.L. Birbeck, L.H. Schwamm, Teleneurology and mobile technologies: The future of neurological care, Nat Rev Neurol. 14 (2018) 285–297. https://doi.org/10.1038/nrneurol.2018.31.
B.H. Dobkin, Behavioral self-management strategies for practice and exercise should be included in neurologic rehabilitation trials and care, Curr Opin Neurol. 29 (2016) 693–699. https://doi.org/10.1097/WCO.0000000000000380.
A Middelweerd, J.S. Mollee, C.N. van der Wal, J. Brug, S.J. te Velde, Apps to promote physical activity among adults: A review and content analysis, Int J Behav Nutr Phys Act. 11 (2014). https://doi.org/10.1186/s12966-014-0097-9.
X. Zhou, M. Du, L. Zhou, Use of mobile applications in post-stroke rehabilitation: a systematic review, Top Stroke Rehabil. 25 (2018) 489–499. https://doi.org/10.1080/10749357.2018.1482446.
M.T. Sánchez Rodríguez, S. Collado Vázquez, P. Martín Casas, R. Cano de la Cuerda, Apps en neurorrehabilitación. Una revisión sistemática de aplicaciones móviles, Neurología. 33 (2018) 313–326. https://doi.org/10.1016/j.nrl.2015.10.005.
M. Linares-del Rey, L. Vela-Desojo, R. Cano-de la Cuerda, Mobile phone applications in Parkinson’s disease: A systematic review, Neurologia. 34 (2019) 38–54. https://doi.org/10.1016/j.nrl.2017.03.006.
M.J. Page, J.E. McKenzie, P.M. Bossuyt, I. Boutron, T.C. Hoffmann, C.D. Mulrow, L. Shamseer, J.M. Tetzlaff, E.A. Akl, S.E. Brennan, R. Chou, J. Glanville, J.M. Grimshaw, A. Hróbjartsson, M.M. Lalu, T. Li, E.W. Loder, E. Mayo-Wilson, S. McDonald, L.A. McGuinness, L.A. Stewart, J. Thomas, A.C. Tricco, V.A. Welch, P. Whiting, D. Moher, The PRISMA 2020 statement: An updated guideline for reporting systematic reviews, The BMJ. 372 (2021). https://doi.org/10.1136/bmj.n71.
A.M. Methley, S. Campbell, C. Chew-Graham, R. McNally, S. Cheraghi-Sohi, PICO, PICOS and SPIDER: A comparison study of specificity and sensitivity in three search tools for qualitative systematic reviews, BMC Health Serv Res. 14 (2014) 1–10. https://doi.org/10.1186/s12913-014-0579-0.
C. Maher, C. Sherrington, R. Herbert, A. Moseley, M. Elkins, Reliability of the PEDro scale for rating quality of randomized controlled trials, Phys Ther. 83 (2003) 713–721. https://doi.org/10.1093/ptj/83.8.713.
N.C. Foley, R.W. Teasell, S.K. Bhogal, M.R. Speechley, Stroke Rehabilitation Evidence-Based Review: methodology, Top Stroke Rehabil. 10 (2003) 1–7. http://www.ncbi.nlm.nih.gov/pubmed/12970828.
J.A.C. Sterne, J. Savović, M.J. Page, R.G. Elbers, N.S. Blencowe, I. Boutron, C.J. Cates, H.Y. Cheng, M.S. Corbett, S.M. Eldridge, J.R. Emberson, M.A. Hernán, S. Hopewell, A. Hróbjartsson, D.R. Junqueira, P. Jüni, J.J. Kirkham, T. Lasserson, T. Li, A. McAleenan, B.C. Reeves, S. Shepperd, I. Shrier, L.A. Stewart, K. Tilling, I.R. White, P.F. Whiting, J.P.T. Higgins, RoB 2: A revised tool for assessing risk of bias in randomised trials, The BMJ. 366 (2019) 1–8. https://doi.org/10.1136/bmj.l4898.
J.J. Deeks, J.P.T. Higgins, D.G. Altman, Analysing data and undertaking meta-analyses, Cochrane Handbook for Systematic Reviews of Interventions. (2019) 241–284. https://doi.org/10.1002/9781119536604.ch10.
M. Asano, B.C. Tai, F.Y.T. Yeo, S.C. Yen, A. Tay, Y.S. Ng, D.A. De Silva, K. Caves, E. Chew, H. Hoenig, G.C. Koh, Home-based tele-rehabilitation presents comparable positive impact on self-reported functional outcomes as usual care: The Singapore Tele-technology Aided Rehabilitation in Stroke (STARS) randomised controlled trial, J Telemed Telecare. 27 (2021) 231–238. https://doi.org/10.1177/1357633X19868905.
K. Hankinson, A. Shaykevich, A.M. Vallence, J. Rodger, M. Rosenberg, C. Etherton-Beer, A Tailored Music-Motor Therapy and Real-Time Biofeedback Mobile Phone App (‘GotRhythm’) to Promote Rehabilitation Following Stroke: A Pilot Study, Neurosci Insights. 17 (2022). https://doi.org/10.1177/26331055221100587.
I. Li, T. Bui, H.T. Phan, A. Llado, C. King, K. Scrivener, App-based supplemental exercise in rehabilitation, adherence, and effect on outcomes: a randomized controlled trial, Clin Rehabil. 34 (2020) 1083–1093. https://doi.org/10.1177/0269215520928119.
P. Ginis, A. Nieuwboer, M. Dorfman, A. Ferrari, E. Gazit, C.G. Canning, L. Rocchi, L. Chiari, J.M. Hausdorff, A. Mirelman, Feasibility and effects of home-based smartphone-delivered automated feedback training for gait in people with Parkinson’s disease: A pilot randomized controlled trial, Parkinsonism Relat Disord. 22 (2016) 28–34. https://doi.org/10.1016/j.parkreldis.2015.11.004.
P. Aphiphaksakul, A. Siriphorn, Home-based exercise using balance disc and smartphone inclinometer application improves balance and activity of daily living in individuals with stroke: A randomized controlled trial, PLoS One. 17 (2022). https://doi.org/10.1371/journal.pone.0277870.
C. Salgueiro, G. Urrútia, R. Cabanas-Valdés, Influence of Core-Stability Exercises Guided by a Telerehabilitation App on Trunk Performance, Balance and Gait Performance in Chronic Stroke Survivors: A Preliminary Randomized Controlled Trial, Int J Environ Res Public Health. 19 (2022). https://doi.org/10.3390/ijerph19095689.
J. Lee, K. Lee, C. Song, Speed-Interactive Treadmill Training Using Smartphone-Based Motion Tracking Technology Improves Gait in Stroke Patients, J Mot Behav. 49 (2017) 675–685. https://doi.org/10.1080/00222895.2016.1271300.
D.C. Shin, C.H. Song, Smartphone-Based Visual Feedback Trunk Control Training Using a Gyroscope and Mirroring Technology for Stroke Patients: Single-blinded, Randomized Clinical Trial of Efficacy and Feasibility, Am J Phys Med Rehabil. 95 (2016) 319–329. https://doi.org/10.1097/PHM.0000000000000447.
M. Grau-Pellicer, J.F. Lalanza, E. Jovell-Fernández, L. Capdevila, Impact of mHealth technology on adherence to healthy PA after stroke: a randomized study, Top Stroke Rehabil. 27 (2020) 354–368. https://doi.org/10.1080/10749357.2019.1691816.
J.H. Kim, S.G. Park, H.J. Lim, G.C. Park, M.H. Kim, B.H. Lee, Effects of the combination of rhythmic auditory stimulation and task-oriented training on functional recovery of subacute stroke patients, J Phys Ther Sci. 24 (2012) 1307–1313. https://doi.org/10.1589/jpts.24.1307.
T.D. Ellis, J.T. Cavanaugh, T. DeAngelis, K. Hendron, C.A. Thomas, M. Saint-Hilaire, K. Pencina, N.K. Latham, Comparative effectiveness of mhealth-supported exercise compared with exercise alone for people with Parkinson disease: Randomized controlled pilot study, Phys Ther. 99 (2019) 203–216. https://doi.org/10.1093/ptj/pzy131.
M. Plow, M. Golding, Using mHealth Technology in a Self-Management Intervention to Promote Physical Activity Among Adults With Chronic Disabling Conditions: Randomized Controlled Trial, JMIR Mhealth Uhealth. 5 (2017) e185. https://doi.org/10.2196/mhealth.6394.
N.N. Nasseri, E. Ghezelbash, Y. Zhai, S. Patra, K. Riemann-Lorenz, C. Heesen, A.C. Rahn, J.P. Stellmann, Feasibility of a smartphone app to enhance physical activity in progressive MS: A pilot randomized controlled pilot trial over three months, PeerJ. 2020 (2020) 1–21. https://doi.org/10.7717/peerj.9303.
M. Matamala-Gomez, M. Maisto, J.I. Montana, P.A. Mavrodiev, F. Baglio, F. Rossetto, F. Mantovani, G. Riva, O. Realdon, The role of engagement in teleneurorehabilitation: A systematic review, Front Neurol. 11 (2020). https://doi.org/10.3389/fneur.2020.00354.
S. Kumar, W.J. Nilsen, A. Abernethy, A. Atienza, K. Patrick, M. Pavel, W.T. Riley, A. Shar, B. Spring, D. Spruijt-Metz, D. Hedeker, V. Honavar, R. Kravitz, R. Craig Lefebvre, D.C. Mohr, S.A. Murphy, C. Quinn, V. Shusterman, D. Swendeman, Mobile health technology evaluation: The mHealth evidence workshop, Am J Prev Med. 45 (2013) 228–236. https://doi.org/10.1016/j.amepre.2013.03.017.
J.E. Harris, J.J. Eng, Goal Priorities Identified through Client-Centred Measurement in Individuals with Chronic Stroke, Physiotherapy Canada. 56 (2004) 171. https://doi.org/10.2310/6640.2004.00017.
S.T. Nemanich, R.P. Duncan, L.E. Dibble, J.T. Cavanaugh, T.D. Ellis, M.P. Ford, K.B. Foreman, G.M. Earhart, Predictors of gait speeds and the relationship of gait speeds to falls in men and women with parkinson disease, Parkinsons Dis. 2013 (2013). https://doi.org/10.1155/2013/141720.
C.D. MacKinnon, Sensorimotor anatomy of gait, balance, and falls, 1st ed., Elsevier B.V., 2018. https://doi.org/10.1016/B978-0-444-63916-5.00001-X.
H.J. Noh, S.H. Lee, D.H. Bang, Three-Dimensional Balance Training Using Visual Feedback on Balance and Walking Ability in Subacute Stroke Patients: A Single-Blinded Randomized Controlled Pilot Trial, Journal of Stroke and Cerebrovascular Diseases. 28 (2019) 994–1000. https://doi.org/10.1016/j.jstrokecerebrovasdis.2018.12.016.
J.G. Rossignol S, Audio-spinal influence in man studied by the H-reflex and its possible role on rhythmic movements synchronized to sound., Electroencephalogr Clin Neurophysiol. 41 (1976) 83–92. https://doi.org/10.1016/0013-4694(76)90217-0.
S. Ghai, I. Ghai, G. Schmitz, A.O. Effenberg, Effect of rhythmic auditory cueing on parkinsonian gait: A systematic review and meta-analysis, Sci Rep. 8 (2018) 1–19. https://doi.org/10.1038/s41598-017-16232-5.
M.H. Thaut, R.R. Rice, T. Braun Janzen, C.P. Hurt-Thaut, G.C. McIntosh, Rhythmic auditory stimulation for reduction of falls in Parkinson’s disease: a randomized controlled study, Clin Rehabil. 33 (2019) 34–43. https://doi.org/10.1177/0269215518788615.
L.R. Nascimento, C.Q. de Oliveira, L. Ada, S.M. Michaelsen, L.F. Teixeira-Salmela, Walking training with cueing of cadence improves walking speed and stride length after stroke more than walking training alone: A systematic review, J Physiother. 61 (2015) 10–15. https://doi.org/10.1016/j.jphys.2014.11.015.
H.A. Miot, Sample size in clinical and experimental, J Vasc Bras. 10 (2011) 275–278. https://doi.org/10.1590/s1677-54492011000400001.
J.K. Tilson, K.J. Sullivan, S.Y. Cen, D.K. Rose, C.H. Koradia, S.P. Azen, P.W. Duncan, Meaningful gait speed improvement during the first 60 days poststroke: minimal clinically important difference, Phys Ther. 90 (2010) 196–208. https://doi.org/10.2522/ptj.20090079.
R.W. Bohannon, A.W. Andrews, S.S. Glenney, Minimal clinically important difference for comfortable speed as a measure of gait performance in patients undergoing inpatient rehabilitation after stroke, J Phys Ther Sci. 25 (2013) 1223–1225. https://doi.org/10.1589/jpts.25.1223.
C.J. Hass, M. Bishop, M. Moscovich, E.L. Stegemöller, J. Skinner, I.A. Malaty, A.W. Shukla, N. McFarland, M.S. Okun, Defining the clinically meaningful difference in gait speed in persons with Parkinson disease, Journal of Neurologic Physical Therapy. 38 (2014) 233–238. https://doi.org/10.1097/NPT.0000000000000055.
U.B. Flansbjer, A.M. Holmbäck, D. Downham, C. Patten, J. Lexell, Reliability of gait performance tests in men and women with hemiparesis after stroke, J Rehabil Med. 37 (2005) 75–82. https://doi.org/10.1080/16501970410017215.
J.M.R. Agnew, C.E. Hanratty, J.G. McVeigh, C. Nugent, D.P. Kerr, An Investigation Into the Use of mHealth in Musculoskeletal Physiotherapy: Scoping Review, JMIR Rehabil Assist Technol. 9 (2022). https://doi.org/10.2196/33609.
A. Gessa, P. Sancha, A. Jiménez, Quality Assessment of Health-apps using a Public Agency Quality and Safety Seal. The Appsaludable Case, J Med Syst. 45 (2021). https://doi.org/10.1007/s10916-021-01748-1.
Acknowledgements
We are grateful to the Spanish Ministry of Science, Innovation and Universities for the scholar grant: FPU19/04377.
Funding
Funding for open access publishing: Universidad de Cádiz/CBUA. This work was supported by the Spanish Ministry of Science, Innovation and Universities [grant number FPU19/04377].
Author information
Authors and Affiliations
Contributions
MML, DLA, AS, JAMM, IF made substantial contributions to the study conception and design; MML, DLA and JAMM conducted the searches and the screening process, and assessed the quality appraisal of the included studies; AS supervised the statistics procedures and interpreted the data. MML, DLA, AS, JAMM, IF drafted the manuscript. All authors have participated sufficiently in the present study and have given the approval of the final version to be published.
Corresponding author
Ethics declarations
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Conflict of interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Moreno-Ligero, M., Lucena-Anton, D., Salazar, A. et al. mHealth Impact on Gait and Dynamic Balance Outcomes in Neurorehabilitation: Systematic Review and Meta-analysis. J Med Syst 47, 75 (2023). https://doi.org/10.1007/s10916-023-01963-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10916-023-01963-y