Abstract
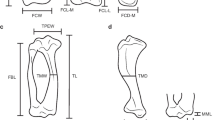
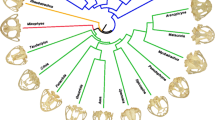
The relationship between ecology and morphology of the limbs in living placental mammals is well established and has been used to infer aspects of palaeobiology for many extinct species. However, few studies have applied these principles to extinct marsupials. Palorchestids are a poorly understood extinct family of megafaunal marsupials of particular interest due to their highly robust and unusual limb morphology. Using a comparative sample of humeri and femora from living mammals potentially analogous to palorchestids, we applied three-dimensional geometric morphometric techniques in a phylogenetic comparative framework. We established that humerus and femur shape in living species showed significant associations with size, phylogeny, and substrate use, and found a weak association between humeral shape and diet. We then examined patterns in morphological disparity, modularity, covariance and morphospace occupation in palorchestids relative to comparative living mammals as well as their closest extinct marsupial relatives. We found palorchestid femora to be unremarkable in shape, while their overall and proximal humeral morphology were strongly divergent from all other mammals sampled. Over their evolutionary history, palorchestid distal humeri increasingly resembled those of mammals adapted for tearing and hook-and-pull digging, while other analyses showed various arboreal-like and fossorial-like affinities in humeral shape. Our findings indicate strong asymmetric selection acting on the fore- and hindlimbs in palorchestids, and their unique combination of shape traits suggests they used their forelimbs in a specialised manner that has no direct equivalence either with their extinct relatives or among other mammals alive today.










Similar content being viewed by others
Data availability
All code and data associated with this study are available in the Online Resources and from Figshare (10.26180/19946171), with specimen 3D meshes available on Morphosource.org where possible (project ID: 000445426).
References
Adams DC (2014) A generalized K statistic for estimating phylogenetic signal from shape and other high-dimensional multivariate data. Syst Biol 63(5):685-697
Adams DC, Collyer M, Kaliontzopoulou A, Baken E (2021) Geomorph: Software for geometric morphometric analyses. R package version 4.0. edn. https://cran.r-project.org/package=geomorph. Accessed 19 Jan 2022
Adams DC, Collyer ML (2018) Phylogenetic ANOVA: Group-clade aggregation, biological challenges, and a refined permutation procedure. Evolution 72(6):1204-1215 https://doi.org/10.1111/evo.13492
Adams DC, Collyer ML (2019) Comparing the strength of modular signal, and evaluating alternative modular hypotheses, using covariance ratio effect sizes with morphometric data. Evolution 73(12):2352-2367 https://doi.org/10.1111/evo.13867
Adams DC, Felice RN (2014) Assessing trait covariation and morphological integration on phylogenies using evolutionary covariance matrices. PLoS ONE 9(4):e94335 https://doi.org/10.1371/journal.pone.0094335
Adams NF, Gray T, Purnell MA (2020) Dietary signals in dental microwear of predatory small mammals appear unaffected by extremes in environmental abrasive load. Palaeogeogr Palaeoclimatol Palaeoecol 558:109929 https://doi.org/10.1016/j.palaeo.2020.109929
Archer M (1984) The Australian marsupial radiation. In: Archer M, Clayton G (eds) Vertebrate Zoogeography and Evolution in Australasia. Hesperian Press, Victoria Park, Australia, pp 633-808
Bardua C, Wilkinson M, Gower DJ, Sherratt E, Goswami A (2019) Morphological evolution and modularity of the caecilian skull. BMC Evol Biol 19(1):1-23
Bargo MS (2003) Biomechanics and palaeobiology of the Xenarthra: the state of the art. Senckenb Biol 83(1):41-50
Beck RMD, Louys J, Brewer P, Archer M, Black KH, Tedford RH (2020) A new family of diprotodontian marsupials from the latest Oligocene of Australia and the evolution of wombats, koalas, and their relatives (Vombatiformes). Sci Rep 10(1):9741 https://doi.org/10.1038/s41598-020-66425-8
Beck RMD, Voss R, Jansa S (2022) Craniodental morphology and phylogeny of marsupials. Bull Am Mus Nat Hist 457:1–350 https://doi.org/10.31233/osf.io/rph78
Bell MA, Lloyd GT (2015) strap: an R package for plotting phylogenies against stratigraphy and assessing their stratigraphic congruence. Palaeontology 58(2):379-389 https://doi.org/10.1111/pala.12142
Black K (2006) Description of new material for Propalorchestes novaculacephalus (Marsupialia: Palorchestidae) from the mid Miocene of Riversleigh, northwestern Queensland. Alcheringa 30(2):351-361
Black K (2008) Diversity, phylogeny and biostratigraphy of diprotodontoids (Marsupialia: Diprotodontidae, Palorchestidae) from the Riversleigh World Heritage Area. Dissertation, University of New South Wales
Blender Online Community (2021) Blender. 2.79 edn. Blender Foundation, Belgium
Blomberg SP, Garland Jr T, Ives AR (2003) Testing for phylogenetic signal in comparative data: Behavioral traits are more labile. Evolution 57(4):717-745
Borcard D, Gillet F, Legendre P (2011) Numerical Ecology with R. Springer, New York
Camens AB, Wells RT (2010) Palaeobiology of Euowenia grata (Marsupialia: Diprotodontinae) and its presence in northern South Australia. J Mamm Evol 17(1):3-19 https://doi.org/10.1007/s10914-009-9121-2
Campione NE, Evans DC (2012) A universal scaling relationship between body mass and proximal limb bone dimensions in quadrupedal terrestrial tetrapods. BMC Biol 10(1):60 https://doi.org/10.1186/1741-7007-10-60
Cascini M (2020) Evolution of Marsupial Biodiversity. Dissertation, Queensland University of Technology
Coombs MC (1983) Large mammalian clawed herbivores: A comparative study. Trans Am Philos Soc 73(7):1-96
Covert HH, Kay RF (1981) Dental microwear and diet: implications for determining the feeding behaviors of extinct primates, with a comment on the dietary pattern of Sivapithecus. Am J Phys Anthropol 55(3):331-336
Davis A, Archer M (1997) Palorchestes azael (Mammalia, Palorchestidae) from the late Pleistocene Terrace Site Local Fauna, Riversleigh, northwestern Queensland. Mem Qld Mus 41:315-320
Den Boer W, Campione NE, Kear BP (2019) Climbing adaptations, locomotory disparity and ecological convergence in ancient stem ‘kangaroos’. R Soc Open Sci 6(2):181617
DeSantis LRG, Field JH, Wroe S, Dodson JR (2017) Dietary responses of Sahul (Pleistocene Australia–New Guinea) megafauna to climate and environmental change. Paleobiology 43(2):1-15 https://doi.org/10.1017/pab.2016.50
Doran DM (1997) Ontogeny of locomotion in mountain gorillas and chimpanzees. J Hum Evol 32(4):323-344 https://doi.org/10.1006/jhev.1996.0095
Etienne C, Filippo A, Cornette R, Houssaye A (2021) Effect of mass and habitat on the shape of limb long bones: A morpho-functional investigation on Bovidae (Mammalia: Cetartiodactyla). J Anat 238(4):886-904 https://doi.org/10.1111/joa.13359
Fabre A-C, Cornette R, Goswami A, Peigné S (2015a) Do constraints associated with the locomotor habitat drive the evolution of forelimb shape? A case study in musteloid carnivorans. J Anat 226(6):596-610 https://doi.org/10.1111/joa.12315
Fabre A-C, Salesa MJ, Cornette R, Antón M, Morales J, Peigné S (2015b) Quantitative inferences on the locomotor behaviour of extinct species applied to Simocyon batalleri (Ailuridae, Late Miocene, Spain). Sci Nat 102(5-6):30
Fariña RA, Vizcaíno SF, Bargo MS (1998) Body mass estimations in Lujanian (late Pleistocene-early Holocene of South America) mammal megafauna. Masozool Neotrop 5(2):87-108
Figueirido B, Martín-Serra A, Janis CM (2016) Ecomorphological determinations in the absence of living analogues: the predatory behavior of the marsupial lion (Thylacoleo carnifex) as revealed by elbow joint morphology. Paleobiology 42(03):508-531 https://doi.org/10.1017/pab.2015.55
Flannery TF, Archer M (1985) Palorchestes: Large and small palorchestids. In: Rich PV, Van Tets GF, Knight F (eds) Kadimakara: Extinct Vertebrates of Australia. Pioneer Design Studio, Melbourne, Victoria, pp 234–239
Fruciano C, Celik MA, Butler K, Dooley T, Weisbecker V, Phillips MJ (2017) Sharing is caring? Measurement error and the issues arising from combining 3D morphometric datasets. Ecol Evol 7(17):7034-7046
Fujiwara S-i, Hutchinson JR (2012) Elbow joint adductor moment arm as an indicator of forelimb posture in extinct quadrupedal tetrapods. Proc R Soc B 279(1738):2561-2570
Gasc J-P (2001) Comparative aspects of gait, scaling and mechanics in mammals. Comp Biochem Physiol, A: Mol Integr Physiol 131(1):121-133 https://doi.org/10.1016/S1095-6433(01)00457-3
Gaubert P, Antunes A, Meng H, Miao L, Peigné S, Justy F, Njiokou F, Dufour S, Danquah E, Alahakoon J, Verheyen E, Stanley WT, O’Brien SJ, Johnson WE, Luo S-J (2018) The complete phylogeny of pangolins: Scaling up resources for the molecular tracing of the most trafficked mammals on earth. J Hered 109(4):347-359 https://doi.org/10.1093/jhered/esx097
Giannini NP, Morales MM, Wilson LAB, Velazco PM, Abdala F, Flores DA (2021) The cranial morphospace of extant marsupials. J Mamm Evol 28(4):1145-1160 https://doi.org/10.1007/s10914-021-09589-y
Guillerme T (2018) dispRity: A modular R package for measuring disparity. Methods Ecol Evol 9(7):1755-1763 https://doi.org/10.1111/2041-210X.13022
Hanot P, Herrel A, Guintard C, Cornette R (2017) Morphological integration in the appendicular skeleton of two domestic taxa: the horse and donkey. Proc R Soc B 284(1864):20171241 https://doi.org/10.1098/rspb.2017.1241
Hayssen V (2011) Tamandua tetradactyla (Pilosa: Myrmecophagidae). Mamm Species 43(875):64-74 https://doi.org/10.1644/875.1
Heath ME (1992a) Manis pentadactyla. Mamm Species (414):1-6 https://doi.org/10.2307/3504143
Heath ME (1992b) Manis temminckii. Mamm Species (415):1-5 https://doi.org/10.2307/3504220
Hedberg CP, Lyons SK, Smith FA (2022) The hidden legacy of megafaunal extinction: Loss of functional diversity and resilience over the Late Quaternary at Hall’s Cave. Global Ecol Biogeogr 31(2):294-307 https://doi.org/10.1111/geb.13428
Hedrick BP, Dickson BV, Dumont ER, Pierce SE (2020) The evolutionary diversity of locomotor innovation in rodents is not linked to proximal limb morphology. Sci Rep 10(717):1-11 https://doi.org/10.1038/s41598-019-57144-w
Hildebrand M (1985) Digging of quadrupeds. In: Hildebrand M, Bramble DM, Liem KF, Wake DB (eds) Functional Vertebrate Morphology. Harvard University Press, Cambridge, pp 89–109
Janis CM, Buttrill K, Figueirido B (2014) Locomotion in extinct giant kangaroos: Were sthenurines hop-less monsters? PLoS ONE 9(10):e109888-e109888 https://doi.org/10.1371/journal.pone.0109888
Janis CM, Martín-Serra A (2020) Postcranial elements of small mammals as indicators of locomotion and habitat. PeerJ 8:e9634 https://doi.org/10.7717/peerj.9634
Janis CM, Napoli JG, Billingham C, Martín-Serra A (2020) Proximal humerus morphology indicates divergent patterns of locomotion in extinct giant kangaroos. J Mamm Evol 27:627-647 https://doi.org/10.1007/s10914-019-09494-5
Johnson CN, Prideaux GJ (2004) Extinctions of herbivorous mammals in the late Pleistocene of Australia in relation to their feeding ecology: No evidence for environmental change as cause of extinction. Austral Ecol 29(5):553-557 https://doi.org/10.1111/j.1442-9993.2004.01389.x
Jones B, Martín-Serra A, Rayfield EJ, Janis CM (2022) Distal humeral morphology indicates locomotory divergence in extinct giant kangaroos. J Mamm Evol 29:27-41 https://doi.org/10.1007/s10914-021-09576-3
Kley N, Kearney M (2007) Adaptations for digging and burrowing. In: Hall BK (ed) Fins Into Limbs: Evolution, Development and Transformation. University of Chicago Press, Chicago and London, pp 284-309
Klingenberg CP (2014) Studying morphological integration and modularity at multiple levels: concepts and analysis. Philos Trans R Soc Lond, Ser B: Biol Sci 369(1649):20130249-20130249 https://doi.org/10.1098/rstb.2013.0249
Kumar S, Stecher G, Suleski M, Hedges SB (2017) TimeTree: a resource for timelines, timetrees, and divergence times. Mol Biol Evol 34(7):1812-1819
López-Aguirre C, Hand SJ, Koyabu D, Tu VT, Wilson LAB (2021) Phylogeny and foraging behaviour shape modular morphological variation in bat humeri. J Anat 238(6):1312-1329 https://doi.org/10.1111/joa.13380
Lungmus JK, Angielczyk KD (2019) Antiquity of forelimb ecomorphological diversity in the mammalian stem lineage (Synapsida). Proc Nat Acad Sci U S A 116(14):6903-6907 https://doi.org/10.1073/pnas.1802543116
Lungmus JK, Angielczyk KD (2021) Phylogeny, function and ecology in the deep evolutionary history of the mammalian forelimb. Proc R Soc B 288(1949):20210494 https://doi.org/10.1098/rspb.2021.0494
Mackness BS (2008) Reconstructing Palorchestes (Marsupialia: Palorchestidae) - from giant kangaroo to marsupial 'tapir'. Proc Linn Soc N S W 130:21-36
MacLaren JA, Nauwelaerts S (2016) A three‐dimensional morphometric analysis of upper forelimb morphology in the enigmatic tapir (Perissodactyla: Tapirus) hints at subtle variations in locomotor ecology. J Morphol 277(11):1469-1485
Mallet C, Billet G, Houssaye A, Cornette R (2020) A first glimpse at the influence of body mass in the morphological integration of the limb long bones: an investigation in modern rhinoceroses. J Anat 237(4):704-726 https://doi.org/10.1111/joa.13232
Martín-Serra A, Figueirido B, Palmqvist P (2014) A three-dimensional analysis of morphological evolution and locomotor performance of the carnivoran forelimb. PLoS ONE 9(1):e85574 https://doi.org/10.1371/journal.pone.0085574
Martín-Serra A, Figueirido B, Palmqvist P (2016) In the pursuit of the predatory behavior of borophagines (Mammalia, Carnivora, Canidae): Inferences from forelimb morphology. J Mamm Evol 23(3):237-249 https://doi.org/10.1007/s10914-016-9321-5
Martín‐Serra A, Figueirido B, Pérez‐Claros JA, Palmqvist P (2015) Patterns of morphological integration in the appendicular skeleton of mammalian carnivores. Evolution 69(2):321-340
Meloro C, de Oliveira AM (2019) Elbow joint geometry in bears (Ursidae, Carnivora): A tool to infer paleobiology and functional adaptations of Quaternary fossils. J Mamm Evol 26:133-146 https://doi.org/10.1007/s10914-017-9413-x
Miljutin A (2009) Substrate utilization and feeding strategies of mammals: description and classification. Estonian J Ecol 58(1):60-71
Milne N, Vizcaíno S, Fernicola J (2009) A 3D geometric morphometric analysis of digging ability in the extant and fossil cingulate humerus. J Zool 278(1):48-56
Mitteroecker P, Gunz P (2009) Advances in geometric morphometrics. Evol Biol 36(2):235-247 https://doi.org/10.1007/s11692-009-9055-x
Montanari S, Louys J, Price GJ (2013) Pliocene paleoenvironments of southeastern Queensland, Australia inferred from stable isotopes of marsupial tooth enamel. PLoS ONE 8(6):e66221
Muñoz NA, Cassini GH, Candela AM, Vizcaíno SF (2017) Ulnar articular surface 3-D landmarks and ecomorphology of small mammals: a case study of two early Miocene typotheres (Notoungulata) from Patagonia. Earth Environ Sci Trans R Soc Edinb 106(4):315-323
Owen R (1869) On the fossil mammals of Australia. Part III. Diprotodon australis, Owen. Philos Trans R Soc Lond, Ser B: Biol Sci 160:519-578
Owen R (1874) On the fossil mammals of Australia. Part 9. Family Macropodidæ; Genera Macropus, Pachysiagon, Leptosiagon, Procoptodon, and Palorchestes. Proc R Soc Lond, Ser B: Biol Sci 164:783-803
Piper KJ (2006) A new species of Palorchestidae (Marsupialia) from the Pliocene and early Pleistocene of Victoria. Alcheringa 30:281-294 https://doi.org/10.1080/03115510609506867
Polly PD (2007) Limbs in mammalian evolution. In: Hall BK (ed) Fins into Limbs. University of Chicago Press, Chicago and London, pp 245-268
R Core Team (2021) R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing. Vienna, Austria
Richards HL, Bishop PJ, Hocking DP, Adams JW, Evans AR (2021) Low elbow mobility indicates unique forelimb posture and function in a giant extinct marsupial. J Anat 238:1425-1441
Richards HL, Wells RT, Evans AR, Fitzgerald EM, Adams JW (2019) The extraordinary osteology and functional morphology of the limbs in Palorchestidae, a family of strange extinct marsupial giants. PLoS ONE 14(9):e0221824
Roberts RG, Flannery TF, Ayliffe LK, Yoshida H, Olley JM, Prideaux GJ, Laslett GM, Baynes A, Smith MA, Jones R (2001) New ages for the last Australian megafauna: continent-wide extinction about 46,000 years ago. Science 292(5523):1888-1892
Samuels JX, Van Valkenburgh B (2008) Skeletal indicators of locomotor adaptations in living and extinct rodents. J Morphol 269(11):1387-1411
Schlager S (2017) Morpho and Rvcg – Shape Analysis in R. In Zheng G, Li S, Szekely G (eds.), Statistical Shape and Deformation Analysis, 217–256. Academic Press. ISBN 9780128104934
Schmidt M, Fischer MS (2009) Morphological integration in mammalian limb proportions: dissociation between function and development. Evolution 63(3):749-766
Scott HH (1915) A monograph of Nototherium tasmanicum (genus-Owen: sp. nov.) Tasman Dep Mines Geol Surv Rec 4:47
Serio C, Raia P, Meloro C (2020) Locomotory adaptations in 3D humerus geometry of Xenarthra: Testing for convergence. Front Ecol Evol 8(139):1-12 https://doi.org/10.3389/fevo.2020.00139
Tarquini J (2021) Femoral shape in procyonids (Carnivora, Procyonidae): Morphofunctional implications, size and phylogenetic signal. J Mamm Evol 28:159-171 https://doi.org/10.1007/s10914-019-09491-8
The Mesquite Project Team (2021) Mesquite: a modular system for evolutionary analysis. 3.70 edn. The Mesquite Project Team. http://www.mesquiteproject.org. Accessed 19 Jan 2022
Toledo N, Muñoz NA, Cassini GH (2021) Ulna of extant xenarthrans: Shape, size, and function. J Mamm Evol 28:35-45 https://doi.org/10.1007/s10914-020-09503-y
Trusler PW, Sharp AC (2016) Description of new cranial material of Propalorchestes (Marsupialia: Palorchestidae) from the middle Miocene Camfield Beds, Northern Territory, Australia. Mem Mus Vic 74:291-324
Villmoare B, Fish J, Jungers W (2011) Selection, morphological integration, and strepsirrhine locomotor adaptations. Evol Biol 38(1):88-99 https://doi.org/10.1007/s11692-011-9108-9
Vizcaíno SF, Toledo N, Bargo MS (2018) Advantages and limitations in the use of extant xenarthrans (Mammalia) as morphological models for paleobiological reconstruction. J Mamm Evol 25(4):496-505 https://doi.org/10.1007/s10914-017-9400-2
Wainwright PC (2007) Functional versus morphological diversity in macroevolution. Annu Rev Ecol Evol Syst 38(1):381-401 https://doi.org/10.1146/annurev.ecolsys.38.091206.095706
Wang Y, Cerling TE (1994) A model of fossil tooth and bone diagenesis: implications for paleodiet reconstruction from stable isotopes. Palaeogeogr Palaeoclimatol Palaeoecol 107(3):281-289
Wilson LAB, Balcarcel A, Geiger M, Heck L, Sánchez-Villagra MR (2021) Modularity patterns in mammalian domestication: Assessing developmental hypotheses for diversification. Evol Lett 5(4):385-396 https://doi.org/10.1002/evl3.231
Woodburne MO (1967) Alcoota Fauna, Central Australia. An integrated palaeontological and geological study. Bull Bur Min Res Geo Geophy Aust 87:1-178
Woods JT (1958) The extinct marsupial genus Palorchestes Owen. Mem Qld Mus 13(4):177-193
Young NM, Hallgrímsson B (2005) Serial homology and the evolution of mammalian limb covariation structure. Evolution 59(12):2691-2704 https://doi.org/10.1111/j.0014-3820.2005.tb00980.x
Zelditch ML, Swiderski DL, Sheets HD (2012) Geometric Morphometrics For Biologists: A Primer. Academic Press, New York and London
Acknowledgements
For access to museum collections, we thank: Sandy Ingleby & Matt McCurry (Australian Museum); Marisa Suvoy, Sara Ketelsen & Eleanor Hoeger (American Museum of Natural History); Adam Yates (Museum and Art Gallery of the Northern Territory); Tim Ziegler, Katie Date, Karen Roberts & Ricky-Lee Erickson (Museums Victoria); Tammy Gordon (Queen Victoria Museum and Art Gallery); Mary-Anne Binnie (South Australian Museum); Rolan Eberhard & Nikki King-Smith (Tasmanian Museum and Art Gallery). We are grateful to Stephanie Pierce and Peter Bishop (Harvard University) for access to Didelphis meshes, Jacob Van Zoelen and Gavin Prideaux for sharing meshes of Kolopsis and Plaisiodon from the MAGNT collection, and to Alex McDonald for CT scanning several extant marsupials used in this study. CT scanning was performed with the valuable assistance of Dr Michael de Veer at Monash Biomedical Imaging. The authors acknowledge the facilities and scientific and technical assistance of the National Imaging Facility (NIF), a National Collaborative Research Infrastructure Strategy (NCRIS) capability at Monash Biomedical Imaging (MBI), a Technology Research Platform at Monash University. Additional meshes were sourced from online databases (Morphosource.org, Sketchfab.com, Digital Morphology Museum KUPRI), detailed in Online Resource 1. HLR thanks Kathleen Garland, Tahlia Pollock and James Rule for their generous advice on data analysis and phylogenetic comparative methods, Laura Wilson for methods assistance and comments on as earlier draft. We are also grateful to Nestor Toledo and an anonymous reviewer for their input that greatly improved this paper.
Funding
This research was supported by an Australian Government Research Training Program Scholarship and Monash University-Museums Victoria PhD Research Scholarship (HLR), internal funds from the Department of Anatomy and Developmental Biology, Monash University (JWA), and the Australian Research Council DP180101797 (ARE).
Author information
Authors and Affiliations
Contributions
HLR led the study, gathered and processed data, performed analyses, constructed figures and drafted the manuscript. HLR, DSR and ARE wrote code and interpreted results. JWA and ARE provided supervision. All authors contributed to and approved the final manuscript text.
Corresponding author
Ethics declarations
Competing interests
The authors have no competing interests to declare that are relevant to the content of this article.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Richards, H.L., Rovinsky, D.S., Adams, J.W. et al. Inferring the palaeobiology of palorchestid marsupials through analysis of mammalian humeral and femoral shape. J Mammal Evol 30, 47–66 (2023). https://doi.org/10.1007/s10914-022-09640-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10914-022-09640-6