Abstract
Mammary gland is an outstanding system to study the regulatory mechanisms governing adult epithelial stem cell activity. Stem cells in the basal layer of the mammary gland fuel the morphogenesis and regeneration of a complex epithelial network during development and upon transplantation. The self-renewal of basal stem/progenitor cells is subjected to regulation by both cell-intrinsic and extrinsic mechanisms. Nfatc1 is a transcription factor that regulates breast tumorigenesis and metastasis, but its role in mammary epithelial development and stem cell function has not been investigated. Here we show that Nfatc1 is expressed in a small subset of mammary basal epithelial cells and its epithelial-specific deletion results in mild defects in side branching and basal-luminal cell balance. Moreover, Nfatc1-deficient basal cells exhibit reduced colony forming ability in vitro and somewhat compromised regenerative potential upon transplantation. Thus, our study provides evidence for a detectable yet non-essential role of Nfatc1 in mammary epithelial morphogenesis and basal stem/progenitor cell self-renewal.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The mammary gland is a dynamic and regenerative organ that undergoes most of its development after birth, with dramatic structural and functional changes occurring during puberty, pregnancy, lactation, and involution. Under the regulation of hormones and local growth factors, stem/progenitor cells in the mammary epithelium self-renew, proliferate, and differentiate to drive the growth, remodeling, and regeneration of the bi-lineage epithelial network composed of an outer basal/myoepithelial layer and an inner luminal layer [1,2,3,4]. The basal compartment houses both unipotent (generating only basal progenies) and multi/bi-potent (generating both basal and luminal progenies) stem cells that fuel morphogenesis and regeneration [1, 5,6,7,8,9]. Despite extensive studies identifying a myriad of molecular and signaling pathways that regulate mammary epithelial stem cell activity and differentiation [1, 6], transcriptional mechanisms underlying mammary epithelial morphogenesis and basal stem cell self-renewal remain to be fully understood.
Nfatc1 (also known as Nfat2) belongs to the NFAT family of transcription factors, which can be dephosphorylated and activated in response to intracellular calcium and translocate into the nucleus to control target gene expression [10]. Nfatc1 plays important roles in both embryonic and adult stem cells, such as regulating early lineage specification in embryonic stem cells [11], maintaining quiescent hair follicle stem cells [12], and directing lung stem cell differentiation and regeneration [13]. Nfatc1 is also required for breast cancer cell migration and invasion in vitro as well as tumorigenesis and metastasis in vivo, and its expression is upregulated in breast cancer [14,15,16]. Nfatc2 protein was reported to accumulate in mammary epithelial cells upon treatment with Wnt5b, an inhibitor of mammary epithelial outgrowth, and was proposed to be a nuclear effector of downstream non-canonical Wnt signaling [17]. Like Nfatc1, Nfatc2 also promotes breast cancer cell invasion and metastasis [16, 18]. However, the in vivo function of Nfat genes in normal mammary gland development and basal cell self-renewal has not been investigated.
In this study, we show that Nfatc1 is expressed in a small subset of mammary basal epithelial cells and its deletion from the mammary epithelium results in mild defects in side branching and basal-luminal cell balance. Moreover, we provide evidence for a detectable but non-essential role for Nfatc1 in basal cell colony formation in vitro and mammary epithelial outgrowth in vivo.
Results
Nfatc1 is Expressed in a Small Subset of Mammary Basal and Luminal Epithelial Cells
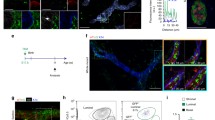
To probe potential Nfatc1 involvement in mammary epithelial morphogenesis, we first interrogated a publicly available microarray dataset on whole mammary gland tissues [19, 20] (see Materials and Methods). This analysis revealed high Nfatc1 expression during early pregnancy that gradually declined by mid-pregnancy and remained low during lactation and involution (Fig. 1a). Using immunostaining, we detected Nfatc1 protein in the basal (marked by basal cell marker K14), luminal (inner, K14− layer), and stromal cells of mammary glands from early pregnant mice (Fig. 1b). Nfatc1-positive cells were rare in the basal and luminal compartments, typically at 1–2 cells per cross-section of a single mammary duct (Fig. 1b). Immunofluorescence and immunohistochemistry also detected Nfatc1-positive basal, luminal, and stromal cells in pubertal mammary glands, both in the ducts and in terminal end bud structures (Fig. 1c, d).
Nfatc1 expression in mouse mammary epithelium. a, Nfatc1 mRNA expression in the mammary gland of wild-type (WT) mice during pregnancy and lactation. Average values of expression were calculated based on raw expression levels of Nfatc1 mRNA detected using Affymetrix MG_U74Av2 chips per GDS2843/102209_at (NCBI's Gene Expression Omnibus). 1 day preg, the day a vaginal plug was observed. N = 4 replicate samples for each stage. b, Immunofluorescence for Nfatc1 protein in the mammary glands of 1-day-pregnant (1 dpc) WT mice (N = 4). Multiple ducts from different mice are shown. c-d, Immunofluorescence (c) and immunohistochemistry (d) for Nfatc1 protein in the mammary glands of 4-week-old WT mice (N = 3). K14 antibody stains the basal cells, and DAPI stains the nuclei. White/red and yellow arrowheads indicate Nfatc1-positive basal and stromal cells, respectively, whereas arrows indicate Nfatc1-positive luminal cells. Note the presence of Nfatc1-positive cap (red arrowhead) and stromal (yellow arrowheads) cells in the terminal end bud structure (d, right). e, RT-qPCR on FACS-isolated basal and luminal cells from 8-week-old virgin WT female mice (N = 5 mice analyzed in different sorting experiments). f, RT-qPCR on sorted basal cells from 15-week-old virgin and mid-pregnant (P14) WT mice. N = 3 mice for each stage. GAPDH gene was used for normalization in e–f. Scale bar: 50 μm in b, c, and d.
We next performed RT-qPCR analysis on FACS-sorted basal and luminal cells of adult virgin mammary glands, which revealed that Nfatc1 mRNA trended towards being expressed at a higher level in basal cells (Figs. 1e and S1a). This finding is consistent with a previous report detecting elevated Nfatc1 expression in mammary basal cells [21], as well as with our own RNA-seq data revealing a threefold enrichment of Nfatc1 mRNA level in basal over luminal cells (p < 10–6) [22]. Compared to FACS-sorted basal cells from adult virgin glands, Nfatc1 mRNA expression decreased significantly in basal cells from mammary glands in mid-pregnancy (Figs. 1f and S1a). We also generated Nfatc1-Cre;ROSAmTmG mice, where Nfatc1-expressing cells and their descendants are marked by GFP expression. Flow cytometry-based quantification of GFP fluorescence in conjunction with basal/luminal surface marker immunostaining revealed rare (< 10%) GFP+ cells in both the basal and luminal populations of 8-week-old virgin and multiparous 6–9-month-old mice (Fig. S1b, c). Together, our data show dynamic Nfatc1 expression in a small subset of basal and luminal epithelial cells of the mammary gland across different reproductive stages.
Epithelium-Specific Deletion of Nfatc1 Results in Mild Reductions in Alveolar Bud Formation/Side Branching and Basal-to-Luminal Cell Ratio
To examine the physiological function of Nfatc1 in mammary epithelial cells, we used K14-Cre as a driver to generate mammary epithelial-specific knockout (MSKO) of Nfatc1 (K14-Cre;Nfatc1f/f). RT-qPCR analysis of control and MSKO basal cells confirmed the reduction of Nfatc1 mRNA expression in the latter (Fig. 2a). At 8 weeks of age, mammary glands from Nfatc1 MSKO mice were similar in morphology to control littermate counterparts (Fig. 2b), suggesting that pubertal mammary development is largely intact. However, by 15 weeks of age, the number of alveolar buds and side branches was significantly reduced in Nfatc1 MSKO mice, whereas the number of major branches was not significantly affected (Fig. 2c, d). This finding suggests a minor role for Nfatc1 in promoting robust mammary epithelial maturation.
Mammary glands of Nfatc1-deficient mice exhibit mild defects at maturation. a, RT-qPCR analysis of sorted mammary basal cells from 15-week-old virgin Nfatc1 MSKO and control (K14-Cre;Nfatc1f/+) littermates. N = 3. b-c, Representative whole-mount carmine staining images of mammary glands from 8-week-old (b) and 15-week-old (c) virgin Nfatc1 MSKO and control (K14-Cre;Nfatc1f/+) littermates. Boxed areas in c are similarly enlarged for control and mutant. d, Quantification of the numbers of alveolar buds/side branches (left) and major branches (right) from N = 3 pairs of 15-week-old virgin Nfatc1 MSKO and control (K14-Cre;Nfatc1f/+ or Nfatc1f/f) littermates. Each dot or square represents a single mouse. Scale bar: 3 mm in b, c.
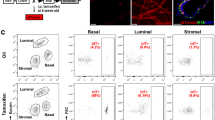
The specific expression of Nfatc1 in basal cells, plus previous report that basal/luminal cell ratio affects side branching [23], led us to wonder whether Nfatc1 deletion affects the balance between basal and luminal cells. While the ratio between flow-detected basal and luminal cell populations was similar in mammary glands from 8-week-old virgin Nfatc1 MSKO and control littermates (Fig. 3a, b), a significant decrease in basal/luminal ratio was observed in mammary glands from 15-week-old Nfatc1 MSKO virgin females compared to control littermates (Fig. 3c, d). Thus, loss of Nfatc1 in the mammary epithelium skews the balance between basal and luminal cells towards the latter, but this alteration is only obvious at mammary epithelial maturation when the morphological defect also manifests.
Nfatc1 deficiency affects mammary basal-luminal balance. Shown are representative flow cytometry profiles (a, c) and quantification of the ratio between basal and luminal cells (b, d) in mammary glands from 8-week-old (a, b) and 15-week-old (c, d) Nfatc1 MSKO and control (Nfatc1f/f or Nfatc1f/+) littermates. N > = 3 pairs. Each dot or square represents a single mouse. Lines between a dot and a square indicate littermates.
Nfatc1 Confers Maximal in Vitro Clonogenicity and in Vivo Regenerative Potential to Mammary Basal Cells
To ask whether Nfatc1 loss specifically impacts mammary basal cells, we performed ex vivo 3D colony formation assays by culturing FACS-sorted basal cells from Nfatc1 MSKO and control littermates in Matrigel [24,25,26,27]. Colonies formed by the Nfatc1 MSKO basal cells were similar in size to those formed by control counterparts both in initial plating and when the cells were serially passaged (Fig. 4a, b). However, at all passages examined, the number of colonies formed in the Nfatc1 MSKO culture was significantly lower than that in the control culture (Fig. 4c). These results suggest that Nfatc1 likely regulates the self-renewal capacity of mammary basal stem/progenitor cells, rather than the rate of basal cell proliferation per se.
Nfatc1-deficient basal cells show significantly reduced colony formation potential in vitro. a, Representative images of colonies derived from FACS-sorted basal cells from 8-week-old Nfatc1 MSKO and control (K14-Cre;Nfatc1f/+, Nfatc1f/f, or Nfatc1f/+) littermates. Scale bar: 500 μm. b-c, Quantification of colony size (b) and number (c) for basal cells derived from N = 3 pairs of Nfatc1 MSKO and control (K14-Cre;Nfatc1f/+, Nfatc1f/f, or Nfatc1f/+) littermates. Colonies from initial plating were harvested and equal numbers (5,000) of Nfatc1 MSKO and control cells were subsequently passaged once (1st passage) or twice (2nd passage). Each dot, square, or triangle represents cells derived from a single mouse.
To complement the in vitro findings above, we conducted transplantation experiments by injecting FACS-sorted basal cells from Nfatc1 MSKO and control littermates into de-epithelialized fat pads of congenic 3-week-old host mice. In three independent experiments where 3,000 Nfatc1 MSKO or control basal cells were injected, the take rate appeared to be minimally impacted (9 out of 9 transplants in control vs. 7 out of 9 in MSKO), but a significant reduction in the percent of fat pad filling was observed in outgrowths derived from the latter (Fig. 5a–c). In separate transplantation experiments using serially decreasing numbers of basal cells from additional pairs of donor mice, the overall take rate was only slightly lower but the average percent of fat pad filling by Nfatc1 MSKO basal cells was again significantly reduced compared to control basal cells (Fig. 5d, e). These results show that Nfatc1 plays a detectable, albeit non-essential, role in promoting the in vivo regenerative potential of mammary basal cells.
Nfatc1-deficient basal cells show reduced regenerative capacity in vivo. a-b, Representative transplants (a) derived from basal cells isolated from 8-week-old Nfatc1 MSKO and control (K14-Cre;Nfatc1f/+, Nfatc1f/f, or Nfatc1f/+) littermates, and summary of take rate (producing a tree that is > 5% of the fat pad) from 3 different experiments (b). Each experiment included 1 pair of Nfatc1 MSKO and control (K14-Cre;Nfatc1f/+, Nfatc1f/f, or Nfatc1f/+) littermate as donor mice, and 3,000 basal cells from each were transplanted onto the contralateral sides of the same host mice. Scale bar: 3 mm. bc, Summary of percent of fat pad filled from (b). d-e, Summary of results from limiting dilution transplantations using FACS-sorted mammary basal cells from additional Nfatc1 MSKO and control (K14-Cre;Nfatc1f/+, Nfatc1f/f, or Nfatc1f/+) littermates. Each pie diagram (d) represents an outgrowth, and data for percent of fat pad filled are summarized in (e). Each dot or square in (c, d) represents a single recipient mouse.
Discussion
Our work has uncovered a role of Nfatc1 in regulating mammary epithelial morphogenesis. The mammary phenotypes caused by epithelial-specific knockout of Nfatc1 are mild, with morphological and basal/luminal ratio defects becoming apparent only in adulthood. As such, Nfatc1 is largely dispensable in the mammary epithelium for ductal elongation and initial branching morphogenesis during pubertal development.
Our findings provide evidence for Nfatc1’s function in promoting mammary basal stem/progenitor cell self-renewal. Of note, even though no morphological defect was detectable in Nfatc1 MSKO mice at 8 weeks of age, basal cells isolated at this developmental stage showed reduced colony forming and regenerative capabilities. This implicates the inherent deficiency of Nfatc1-deficient basal cells that can be unmasked when cells are taken out of their endogenous tissue environment, consistent with the prevailing theme of differential basal stem cell potential under physiological vs. heterogeneous environments [5, 7,8,9, 28, 29]. Nfatc1-deficient basal cells may lose their self-renewal capability because of compromised cell adhesion, basal gene expression, and/or proliferation potential. Future work is needed to address these possibilities.
It is somewhat surprising that Nfatc1 expression in such a small number of basal cells (~ 2% at 8 weeks) in vivo translates into a detectable minor branching phenotype. It is possible that Nfatc1-expressing basal cells might represent a pool of stem/progenitor cells, where Nfatc1 functions cell-autonomously, in the basal compartment that contribute to side branching and alveolar bud formation. Lineage tracing analysis using Nfatc1-CreER mice, which lies outside the scope of the current work, will be able to track the fate of the Nfatc1-expressing basal cells during mammary epithelial development and regeneration. Alternatively, Nfatc1 might regulate the expression of genes encoding secreted factors, which in turn impact a large number of cells including the non-Nfatc1-expressing basal cells.
Our findings add Nfatc1 to the growing list of regulatory factors that govern the activities of mammary basal stem/progenitor cells. High levels of NFATc1 expression in breast cancer correlate with poor prognosis [14]. The functional involvement of Nfatc1 in normal mammary basal stem/progenitor cells raises the possibility that NFATc1 may also regulate the activity of malignant stem/progenitor cells in breast cancer, a notion worth testing in the future.
Materials and Methods
Mice
Nfatc1f/f mice [30] were purchased from the Jackson Laboratory (Stock # 022786) and bred with K14-Cre mice [31, 32] to generate mammary epithelial-specific knockout (MSKO) of Nfatc1. Nfatc1-Cre mice were as previously reported [33], and were bred with ROSAmTmG mice the from the Jackson Laboratory (Stock # 007576). Primers used for genotyping are listed in Table S1. Control and mutant littermates were housed in the same cage before analysis to synchronize the estrous cycles [34]. All mouse experiments have been approved by and conform to the regulatory guidelines of the International Animal Care and Use Committee of the University of California, Irvine.
Isolation of Mammary Epithelial Cells
Single-cell preparation of mammary epithelial cells was performed as previously described [22, 32]. Briefly, mammary glands were isolated from 8-week-old virgin or other specified-stage female mice and placed in the digestion mix [DMEM/F12 (1:1) with 5% FBS, 300 U/mL collagenase (Sigma, C9891) and 100 U/mL hyaluronidase (Sigma, H3506)] for 1.5 h at 37 °C. Cells were pelleted and resuspended in red blood cell lysis buffer (Sigma, R7757). Single cell suspension was obtained by further treatment with 0.25% trypsin–EDTA (Gibco, 25200), 10 mg/mL DNase (Sigma, DN25), and 5 mg/mL dispase (Stem Cell Technologies, 07913), followed by filtration using a 40 μm-pore mesh filter (SWiSH, TC70-MT-1).
Cell Labeling and Flow Cytometry
Single cell suspension from above was stained using the following antibodies and reagents: anti-CD49f-FITC (1:250, Bio Legend, 102205), anti-EpCAM-PE-Cy7 (1:250, Bio Legend, 118215), anti-lineage-APC [1:250; including APC-CD45 (BD Biosciences, 559864), APC-CD31 (BD Biosciences, 551262), APC-TER119 (BD Biosciences, 557909)], and SytoxBlue (Invitrogen, S3457). Flow cytometry analysis and sorting were performed on a FACSAria (Becton Dickenson UK).
Gene Expression Analysis
Total RNA was extracted from the FACS-sorted cells using RNeasy Mini Kit with on-column DNase treatment according to manufacturer’s protocol (QIAGEN). cDNA was synthesized using High-Capacity cDNA Reverse Transcription Kit (Thermo Fisher Scientific) according to manufacturer’s instructions. Real-time PCR was performed using a SYBR Green Supermix (BioRad) on a CFX96 RT-qPCR system, and data were analyzed using the 2 − ΔΔCT method. Primers used for qPCR are listed in Table S1. Analysis of Nfatc1 expression during pregnancy and lactation was based on a published microarray dataset (https://www.ncbi.nlm.nih.gov/geoprofiles/42110332) on mammary gland samples from FVB mice [19, 20].
Immunohistochemistry and Mammary Gland Whole-mount Analysis
Immunohistochemistry was performed as previously described [32]. For whole-mount analysis, the #4 pair of mammary glands were dissected and fixed in Carnoy’s fixative (10% acetic acid, 30% CHCl3, 60% ethanol) for 2–4 h. Fixed tissues were treated with a gradient of ethanol (100%, 70%, 30%) and then washed with sterile water for 10 min. Tissues were then incubated with carmine-alum staining solution as previously described [35]. Images were captured using a Keyence microscope.
Cleared Fat Pad Transplantation
Fat pad clearing and transplantation was as previously described [32]. Briefly, single cell suspensions of sorted basal cells from Nfatc1 MSKO and control littermates were prepared as described above and diluted in a 1:1 solution of 5% FBS media/Matrigel at a concentration of 2,000 cells/10 μL. Ten μL of the cell/Matrigel solution was injected into cleared fat pads of #4 mammary glands of 3-week-old C57BL/6 females, with each host mouse receiving contralateral injections of MSKO and control samples. Outgrowths were analyzed 8–9-weeks later using whole-mount carmine alum staining.
Colony Formation
Sorted basal cells were resuspended in a chilled 1:1 solution of EpiCult-B medium (Stem Cell Technologies)/growth factor-reduced Matrigel (BD Biosciences), and 5,000 cells were plated into one well from 8-well chamber slides (Thermo Fisher Scientific). After Matrigel hardens, 400 μl of EpiCult-B medium containing 10 ng/mL EGF (Millipore, 01–107), 10 ng/mL bFGF (PeproTech, 100-18B), and 4 μg/mL heparin (Stem Cell Technologies, 07980) was added into each well. Culture medium was changed every three days for two weeks, followed by counting colony number and measuring colony size. To passage the colonies, medium was first removed and Matrigel dissolved using 200 μL of dispase for at least 20 min. Single cells were obtained by incubation in 0.25% Trypsin–EDTA followed by filtration using a 40 μm filter, and 5,000 cells were then re-plated as described above.
Statistics
Independent experiments were performed on at least three biological replicates. The sample sizes are indicated in the relevant figure legends. For analysis of differences between groups, Student’s paired t-test was performed with Prism. p values of 0.05 or less were considered statistically significant. Error bars in figures represent mean ± standard error of the mean (SEM).
References
Fu NY, Nolan E, Lindeman GJ, Visvader JE. Stem cells and the differentiation hierarchy in mammary gland development. Physiol Rev. 2020.
Macias H, Hinck L. Mammary gland development. Wiley Interdiscip. Rev Dev Biol. 2012.
Sternlicht MD, Kouros-Mehr H, Lu P, Werb Z. Hormonal and local control of mammary branching morphogenesis. Differentiation. 2006.
Watson CJ, Khaled WT. Mammary development in the embryo and adult: A journey of morphogenesis and commitment. Development. 2008.
Rios AC, Fu NY, Lindeman GJ, Visvader JE. In situ identification of bipotent stem cells in the mammary gland. Nature. 2014.
Visvader JE, Stingl J. Mammary stem cells and the differentiation hierarchy: Current status and perspectives. Genes Dev. 2014.
Van Keymeulen A, Rocha AS, Ousset M, Beck B, Bouvencourt G, Rock J, et al. Distinct stem cells contribute to mammary gland development and maintenance. Nature. 2011.
Shackleton M, Vaillant F, Simpson KJ, Stingl J, Smyth GK, Asselin-Labat ML, et al. Generation of a functional mammary gland from a single stem cell. Nature. 2006.
Stingl J, Eirew P, Ricketson I, Shackleton M, Vaillant F, Choi D, et al. Purification and unique properties of mammary epithelial stem cells. Nature. 2006.
Crabtree GR, Olson EN. NFAT signaling: Choreographing the social lives of cells. Cell. 2002.
Li X, Zhu L, Yang A, Lin J, Tang F, Jin S, et al. Calcineurin-NFAT signaling critically regulates early lineage specification in mouse embryonic stem cells and embryos. Cell Stem Cell. 2011.
Horsley V, Aliprantis AO, Polak L, Glimcher LH, Fuchs E. NFATc1 Balances Quiescence and Proliferation of Skin Stem Cells. Cell. 2008.
Lee JH, Bhang DH, Beede A, Huang TL, Stripp BR, Bloch KD, et al. Lung stem cell differentiation in mice directed by endothelial cells via a BMP4-NFATc1-thrombospondin-1 axis. Cell. 2014.
Kang X, Wang S, Song Y. NFATc1 protein expression and its relationship with clinical characteristics in breast cancer. J Clin Oncol. 2012.
Oikawa T, Nakamura A, Onishi N, Yamada T, Matsuo K, Saya H. Acquired expression of NFATc1 downregulates e-cadherin and promotes cancer cell invasion. Cancer Res. 2013.
Tran Quang C, Leboucher S, Passaro D, Fuhrmann L, Nourieh M, Vincent-Salomon A, et al. The calcineurin/NFAT pathway is activated in diagnostic breast cancer cases and is essential to survival and metastasis of mammary cancer cells. Cell Death Dis. 2015.
Kessenbrock K, Dijkgraaf GJP, Lawson DA, Littlepage LE, Shahi P, Pieper U, et al. A Role for matrix metalloproteinases in regulating mammary stem cell function via the Wnt signaling pathway. Cell Stem Cell. 2013.
Yiu GK, Toker A. NFAT induces breast cancer cell invasion by promoting the induction of cyclooxygenase-2. J Biol Chem. 2006.
Rudolph MC, McManaman JL, Hunter L, Phang T, Neville MC. Functional development of the mammary gland: Use of expression rofiling and trajectory clustering to reveal changes in gene expression during pregnancy, lactation, and involution. J Mammary Gland Biol Neoplasia. 2003.
Anderson SM, Rudolph MC, McManaman JL, Neville MC. Key stages in mammary gland development. Secretory activation in the mammary gland: It’s not just about milk protein synthesis!. Breast Cancer Res. 2007.
Soady KJ, Kendrick H, Gao Q, Tutt A, Zvelebil M, Ordonez LD, et al. Mouse mammary stem cells express prognostic markers for triple-negative breast cancer. Breast Cancer Res. 2015.
Gu B, Watanabe K, Sun P, Fallahi M, Dai X. Chromatin effector Pygo2 mediates wnt-notch crosstalk to suppress luminal/alveolar potential of mammary stem and basal cells. Cell Stem Cell. 2013;13.
Macias H, Moran A, Samara Y, Moreno M, Compton JE, Harburg G, et al. SLIT/ROBO1 signaling suppresses mammary branching morphogenesis by limiting basal cell number. Dev Cell. 2011.
Jardé T, Lloyd-Lewis B, Thomas M, Kendrick H, Melchor L, Bougaret L, et al. Wnt and Neuregulin1/ErbB signalling extends 3D culture of hormone responsive mammary organoids. Nat Commun. 2016.
Gu B, Watanabe K, Sun P, Fallahi M, Dai X. Chromatin effector Pygo2 mediates wnt-notch crosstalk to suppress luminal/alveolar potential of mammary stem and basal cells. Cell Stem Cell. 2013;13:48–61.
Watanabe K, Villarreal-Ponce A, Sun P, Salmans ML, Fallahi M, Andersen B, et al. Mammary morphogenesis and regeneration require the inhibition of EMT at terminal end buds by ovol2 transcriptional repressor. Dev Cell. 2014;29.
Kleinman HK, Martin GR. Matrigel: Basement membrane matrix with biological activity. Semin Cancer Biol. 2005.
Prater MD, Petit V, Alasdair Russell I, Giraddi RR, Shehata M, Menon S, et al. Mammary stem cells have myoepithelial cell properties. Nat Cell Biol. 2014.
Van Amerongen R, Bowman AN, Nusse R. Developmental stage and time dictate the fate of Wnt/β-catenin- responsive stem cells in the mammary gland. Cell Stem Cell. 2012.
Aliprantis AO, Ueki Y, Sulyanto R, Park A, Sigrist KS, Sharma SM, et al. NFATc1 in mice represses osteoprotegerin during osteoclastogenesis and dissociates systemic osteopenia from inflammation in cherubism. J Clin Invest. 2008.
Gu B, Sun P, Yuan Y, Moraes RC, Li A, Teng A, et al. Pygo2 expands mammary progenitor cells by facilitating histone H3 K4 methylation. J Cell Biol. 2009;185:811–26.
Watanabe K, Villarreal-Ponce A, Sun P, Salmans ML, Fallahi M, Andersen B, et al. Mammary morphogenesis and regeneration require the inhibition of EMT at terminal end buds by ovol2 transcriptional repressor. Dev Cell [Internet]. Elsevier Inc.; 2014;29:59–74. Available from: https://doi.org/10.1016/j.devcel.2014.03.006.
Wu B, Zhang Z, Lui W, Chen X, Wang Y, Chamberlain AA, et al. Endocardial cells form the coronary arteries by angiogenesis through myocardial-endocardial VEGF signaling. Cell. 2012.
Handelmann G, Ravizza R, Ray WJ. Social dominance determines estrous entrainment among female hamsters. Horm Behav. 1980.
Sympson CJ, Talhouk RS, Alexander CM, Chin JR, Clift SM, Bissell MJ, et al. Targeted expression of stromelysin-1 in mammary gland provides evidence for a role of proteinases in branching morphogenesis and the requirement for an intact basement membrane for tissue-specific gene expression. J Cell Biol. 1994.
Acknowledgements
We thank the Institute for Immunology FACS Core Facility at the University of California, Irvine for expert service. This work was supported by NIH Grants R01-GM123731 and R01-AR068074 (X.D.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors have no conflict of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Melissa McNeil and Yingying Han have contributed equally to this work.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
McNeil, M., Han, Y., Sun, P. et al. Nfatc1’s Role in Mammary Epithelial Morphogenesis and Basal Stem/progenitor Cell Self-renewal. J Mammary Gland Biol Neoplasia 26, 357–365 (2021). https://doi.org/10.1007/s10911-021-09502-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10911-021-09502-6