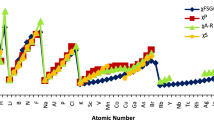
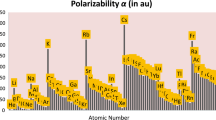
Abstract
Chemistry plays a vital role in the improvement of the narrative properties of atoms and molecules. Many attempts have been made in this field and a few quantities like electronegativity, electron affinity, electrophilicity, polarizability, ionization potential, etc. have been introduced. Electronegativity is an important chemical construct that plays an essential role to clarify several chemical, biochemical, and physicochemical interactions. We have thoroughly studied the different periodic descriptors which are involved to define various electronegativity scales based on either theoretical concepts or experimental findings. The Floating Spherical Gaussian Orbital Approach (FSGO), being one of the the most studied ab initio methods, has been utilized in this study to develop a scale of electronegativity in terms of compressibility factor. We have proposed a model to compute atomic electronegativity values of 51 elements of the periodic table. The computed electronegativity scale observes the periodic trend and justifies many chemical phenomena. Molecular electronegativity values have also been computed using the computed atomic electronegativity data and utilized to verify the Electronegativity Equalization Principle.





Similar content being viewed by others
Data availability
The data that supports the findings of this study are available within the article and its supplementary material.
References
A.A. Frost, Floating spherical gaussian orbital model of molecular structure. II. One-and two-electron-pair systems. J. Chem. Phys. 47(10), 3714–4371 (1967)
G.M. Maggiora, J.D. Petke, R.E. Christoffersen, Electronic excited states of biomolecular systems: ab initio FSGO-based quantum mechanical methods with applications to photosynthetic and related systems, in Theoretical Treatment of Large Molecules and Their Interactions: Part 4 Theoretical Models of Chemical Bonding (1991), pp. 65–102.
A.A. Frost, A floating spherical Gaussian orbital model of molecular structure. III. First-row atom hydrides. J. Phys. Chem. 72(4), 1289–1293 (1968)
A.A. Frost, R.A. Rouse, Floating spherical Gaussian orbital model of molecular structure. IV. Hydrocarbons. J. Am. Chem. Soc. 90(8), 1965–1969 (1968)
S.Y. Chu, A.A. Frost, Floating spherical gaussian orbital model of molecular structure. IX. Diatomic molecules of first-row and second-row atoms. J. Chem. Phys. 54(2), 764–768 (1971)
R.A. Suthers, J.W. Linnett, On the use of minimal basis sets of gaussian-type orbitals. Chem. Phys. Lett. 29(4), 589–593 (1974)
W.D. Erickson, J.W. Linnett, Gaussian orbital calculations of solids. Crystalline lithium hydride. J. Chem. Soc. Faraday Trans. II 68, 693–710 (1972)
W.D. Erickson, J.W. Linnett, An ‘ab initio’Gaussian orbital calculation of the (100) surface of crystalline lithium hydride. Proc. Math. Phys. Eng. 331(1586), 347–359 (1972)
W. Schulz, W. Gruendler, Phys. Status Solidi B 78, 18 (1976)
W. Schulz, W. Gruendler, Zeit fuer Phys. Chemie 259, 889 (1978)
J.L. Nelson, A.A. Frost, A floating spherical Gaussian orbital model of molecular structure. ESCA chemical shifts for inner shell electrons for small hydrocarbons. Chem. Phys. Lett. 13(6), 610–612 (1972)
P.A. Cox, S. Evans, A.F. Orchard, N.V. Richardson, P.J. Roberts, Simple quantitative molecular orbital methods used in connection with photoelectron spectroscopy. Faraday Discuss 54, 26–40 (1972)
T.J. O’Donnell, P.R. LeBreton, L.L. Shipman, Ab initio quantum mechanical characterization of the ground electronic state of uracil. J. Phys. Chem. 82(3), 343–347 (1978)
A.M. Semkow, J.W. Linnett, Applications of a simple molecular wavefunction. Part 15.—Spectroscopic constants of selected first-row diatomic hydride molecules. J. Chem. Soc. Faraday Trans. II 72, 1503–1512 (1976)
J.M. André, J. Delhalle, J.J. Pireaux, Band Structure Calculations and Their Relations to Photoelectron Spectroscopy (ACS Publications, Columbus, 1981)
J. Szaniszlo, I. Tamassy-Lentei, Acta Phys. Chim. Debrecina 33, 43 (2000)
Tamassy-Lentei I, J Szaniszlo J (1976) Acta Physica et Chimica Debrecina 20:59
J. Szaniszlo, I. Tamassy-Lentei, Acta Phys. Chim. Debrecina 36, 65 (2003)
N.K. Ray, Chem. Phys. Lett. 6, 225 (1970)
S. Bhargava, A.K. Bakhshi, N.K. Ray, Indian J. Chem. Sec. A 19A, 1203 (1981)
J.M. Andre, G. Hardy, D.H. Mosley, L. Piela, Top. Mol. Org. Eng. 14, 189 (1996)
W. Gründler, T. Steinke, P. Walther, H/He molecules in strong electric fields. J. Compu.t Chem. 11(5), 548–559 (1990)
J.M. Andre, J. Delhalle, J.G. Fripiat, G. Hennico, L. Piela, Int. J. Quantum Chem. Symp. 22, 665 (1988)
L. Pauling, The nature of the chemical bond. IV. The energy of single bonds and the relative electronegativity of atoms. J. Am. Chem .Soc. 54(9), 3570–3582 (1932)
A.L. Allred, E.G. Rochow, A scale of electronegativity based on electrostatic force. J. Inorg. Nucl. 5(4), 264–268 (1958)
R.S. Mulliken, A new electroaffinity scale; together with data on valence states and on valence ionization potentials and electron affinities. J. Chem. Phys. 2(11), 782–793 (1934)
R.T. Sanderson, Principles of electronegativity Part I. General nature. J. Chem. Educ. 65(2), 112 (1988)
P. Politzer, Z. Peralta-Inga Shields, F.A. Bulat, J.S. Murray, Average local ionization energies as a route to intrinsic atomic electronegativities. J. Chem. Theory Comput 7(2), 377–384 (2011)
P. Politzer, J.S. Murray, Electronegativity: a continuing enigma. J. Phys. Org. Chem. 2022, e4406 (2022)
V. Kumari, T. Singh, S. Devi, H. Tandon, M. Labarca, T. Chakraborty, Atomic electronegativity based on hardness and floating spherical gaussian orbital approach. J. Math. Chem. 1, 1–13 (2022)
K.D. Saloni, H. Tandon, M. Labarca, T. Chakraborty, Computation of atomic electronegativity values using atomic and covalent potential: a FSGO based study. J. Math. Chem. 60(8), 1505–1520 (2022)
D. Kumari, H. Tandon, M. Labarca, T. Chakraborty, An FSGO based electronegativity scale invoking the electrophilicity index. ChemistrySelect 7(44), e202202238 (2022)
D. Xue, S. Zuo, H. Ratajczak, Electronegativity and structural characteristics of lanthanides. Phys. B 352(1–4), 99–104 (2004)
S.G. Hur, T.W. Kim, S.J. Hwang, H. Park, W. Choi, S.J. Kim, J.H. Choy, Synthesis of new visible light active photocatalysts of Ba (In1/3Pb1/3M1/3 ‘) O3 (M ‘= Nb, Ta): a band gap engineering strategy based on electronegativity of a metal component. J. Phys. Chem. B 109(31), 15001–15007 (2005)
D.R. Herrick, Connecting pauling and mulliken electronegativities. J. Chem. Theory Comput. 1(2), 255–260 (2005)
D.V. Louzguine-Luzgin, A. Inoue, W.J. Botta, Reduced electronegativity difference as a factor leading to the formation of Al-based glassy alloys with a large supercooled liquid region of 50 K. Appl. Phys. Lett. 88(1), 011911 (2006)
S. Noorizadeh, E. Shakerzadeh, Bond dissociation energies from a new electronegativity scale. J. Mol. Struct. 20(1–3), 110–113 (2009)
H. Tandon, T. Chakraborty, V. Suhag, A scale of atomic electronegativity in terms of atomic nucleophilicity index. Found. Chem. 22, 335–346 (2020)
S. Carniato, L. Journel, R. Guillemin, M.N. Piancastelli, W.C. Stolte, D.W. Lindle, M. Simon, A new method to derive electronegativity from resonant inelastic x-ray scattering. J. Chem. Phys. 137(14), 144303 (2012)
S. Kaya, C. Kaya, A new equation based on ionization energies and electron affinities of atoms for calculating of group electronegativity. Comput. Theor. Chem. 1052, 42–46 (2015)
A. Qteish, Electronegativity scales and electronegativity-bond ionicity relations: a comparative study. J. Phys. Chem. Solids 124, 186–191 (2019)
L.P. Williams, André-Marie Ampère. Sci. Am. 260(1), 90–97 (1989)
S. Noorizadeh, M. Parhizgar, The atomic and group compressibility. J. Mol. Struct. 725(1–3), 23–26 (2005)
J.P. Connerade, R. Semaoune, Atomic compressibility and reversible insertion of atoms into solids. J. Phys. B 33(17), 3467 (2000)
H. Tandon, T. Chakraborty, V. Suhag, A model of atomic compressibility and its application in QSAR domain for toxicological property prediction. J. Mol. Model. 25, 1–14 (2019)
J. Hinze, H.H. Jaffe, Electronegativity. I. Orbital electronegativity of neutral atoms. J. Am. Chem. Soc. 84(4), 540–546 (1962)
W. Gordy, A new method of determining electronegativity from other atomic properties. Phys. Rev. 69(11–12), 604 (1946)
C. Tantardini, A.R. Oganov, Thermochemical electronegativities of the elements. Nat. Commun. 12(1), 2087 (2021)
R.T. Sanderson, An interpretation of bond lengths in alkali halide gas molecules. J. Am. Chem. Soc. 74(1), 272–274 (1952)
R.A. Donnelly, R.G. Parr, Elementary properties of an energy functional of the first-order reduced density matrix. J. Chem. Phys. 69(10), 4431–4439 (1978)
P. Politzer, H. Weinstein, Some relations between electronic distribution and electronegativity. J. Chem. Phys. 71(11), 4218–4220 (1979)
R.G. Parr, L.J. Bartolotti, On the geometric mean principle for electronegativity equalization. J. Am. Chem. Soc. 104(14), 3801–3803 (1982)
R.F. Nalewajski, A study of electronegativity equalization. J. Phys. Chem. 89(13), 2831–2837 (1985)
P.K. Chattaraj, S. Giri, S. Duley, Electrophilicity equalization principle. J. Phys. Chem. Lett. 1(7), 1064–1067 (2010)
R.G. Parr, Companions in the search. Int. J. Quantum Chem. 49, 739–770 (1994)
R.A. Miranda-Quintana, P.W. Ayers, The “| Δμ| big is good” rule, the maximum hardness, and minimum electrophilicity principles. Theor. Chem. Acc. 138, 1–6 (2019)
R.A. Miranda-Quintana, P.W. Ayers, F. Heidar-Zadeh, Reactivity and charge transfer beyond the parabolic model: the “| Δμ| big is good” principle. Chem. Select. 6(1), 96–100 (2021)
R.A. Miranda-Quintana, A kinetic perspective of charge transfer reactions: the downfall of hard/soft acid/base interactions. Theor. Chem. Acc. 142(5), 52 (2023)
U. Hohm, Is there a minimum polarizability principle in chemical reactions? J. Phys. Chem. A 104(36), 8418–8423 (2000)
H. Tandon, T. Chakraborty, V. Suhag, On the validity of minimum magnetizability principle in chemical reactions. Acta Chim. Slov. 68(1), 178–184 (2021)
R.A. Miranda-Quintana, F. Heidar-Zadeh, P.W. Ayers, Elementary derivation of the “| Δμ| big is good” rule. J. Phys. Chem. Lett. 9(15), 4344–4348 (2018)
R.A. Miranda-Quintana, P.W. Ayers, Dipolar cycloadditions and the “| Δ μ| big is good” rule: a computational study. Theor. Chem. Acc. 137, 1–7 (2018)
L.C. Allen, J.F. Capitani, G.A. Kolks, G.D. Sproul, Van Arkel—Ketelaar triangles. J. Mol. Struct. 300, 647–655 (1993)
W.B. Jensen, A quantitative van Arkel diagram. J. Chem. Educ. 72(5), 395 (1995)
G. Sproul, Electronegativity and bond type: predicting bond type. J. Chem. Educ. 78(3), 387 (2001)
A.E.V. Arkel, Molecules and Crystals in Inorganic Chemistry. Read Books Limited (2011)
T.L. Meek, L.D. Garner, Electronegativity and the bond triangle. J. Chem. Educ. 82(2), 325 (2005)
Acknowledgements
Ms. Dimple Kumari, Ms. Saloni, and Dr. Tanmoy Chakraborty are thankful to Sharda University for providing the research facility.
Funding
Dr. Tanmoy Chakraborty acknowledges the funding support from the Science and Engineering Research Board (SERB), Department of Science and Technology, Government of India, under Grant No. CRG/2020/002951. Dr. Martín Labarca is thankful to Agencia Nacional de PromocíonCientífica y Tecnológica (FONCyT) (Grant PICT-2018-04519), to Universidad de Buenos Aires (Grant UBACyT 20020190200097BA) and to Universidad Austral of Argentina.
Author information
Authors and Affiliations
Contributions
TC conceptualized this study. Data collection, and analysis were performed by DK. The first draft of manuscript was written by DK and SSaloni. Supervision, writing—review and editing done by ML. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declared there were no financial interests or individual relationships to impact the published paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kumari, D., Saloni, S., Labarca, M. et al. Application of floating spherical Gaussian orbital approach in redefining the atomic periodic descriptor. J Math Chem 61, 1924–1935 (2023). https://doi.org/10.1007/s10910-023-01494-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10910-023-01494-4