Abstract
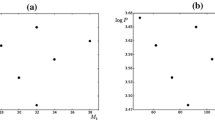
The recent work has an aim to establish a pivotal role of orbital exponent in the normalized atomic radii, atomic density and atomic hardness. These three periodic descriptors help to understand the real scenario of an element. Concerning the effective nuclear charge, screening constant and effective principal quantum number, we have developed a new relation between these periodic properties and invoked a new formula by which we can compute the normalized radii, density and global atomic hardness in terms of the orbital exponent. With comparison to the existing famous formulae originating from different concepts, we can conclude that our empirical computation has an inherent efficacy to predict periodicity.










Similar content being viewed by others
References
D. Ghosh, R. Biswas, Theoretical calculation of absolute radii of atoms and ions. Part 1. The atomic radii. Int. J. Mol. Sci. 3(2), 87–113 (2002)
S.T.K. Gazi, D.C. Ghosh, Computation of the atomic radii through the conjoint action of the effective nuclear charge and the ionisation energy. Mol. Phys. 108(16), 2081–2092 (2010)
L. Pauling, The Nature of the Chemical Bond, vol. 260 (Cornell University Press, Ithaca, 1960), pp. 3175–3187
B. Cordero, V. Gómez, A.E. Platero-Prats, M. Revés, J. Echeverría, E. Cremades, S. Alvarez, Covalent radii revisited. Dalton Trans. 21, 2832–2838 (2008)
R.T. Shannon, C.T. Prewitt, Effective ionic radii in oxides and fluorides. Acta Crystallogr. Sect. B Struct. Crystallogr. Cryst. Chem. 25(5), 925–946 (1969)
R.D. Shannon, Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. Section A Cryst. Phys. Diffract. Theor. General Crystallogr 32(5), 751–767 (1976)
J.T. Waber, D.T. Cromer, Orbital radii of atoms and ions. J. Chem. Phys. 42(12), 4116–4123 (1965)
P. Politzer, R.G. Parr, D.R. Murphy, Relationships between atomic, chemical potentials, electrostatic potentials, and covalent radii. J. Chem. Phys. 79(8), 3859–3861 (1983)
B.M. Deb, P.K. Chattaraj, New quadratic nondifferential Thomas-Fermi-Dirac-type equation for atoms. Phys. Rev. A 37(10), 4030 (1988)
S. Nath, S. Bhattacharya, P.K. Chattaraj, Density functional calculation of a characteristic atomic radius. J. Mol. Struct. (Thoechem) 331(3), 267–279 (1995)
J.E. Huheey, E.A. Keiter, R.L. Keiter, O.K. Medhi, Inorganic Chemistry: Principles of Structure and Reactivity (Pearson Education, India, 2006).
R.T. Sanderson, The covalent radius of xenon. Inorg. Chem. 2(3), 660–661 (1963)
R.T. Sanderson, The covalent radius of radon and the electronegativities of gold, mercury, thallium, lead, and bismuth. J. Inorg. Nuclear Chem. 7, 288 (1958)
T.L. Meek, Electronegativities of the noble gases. J. Chem. Educ. 72(1), 17 (1995)
A. Bondi, van der Waals volumes and radii. J. Phys. Chem. 68(3), 441–451 (1964)
P.G. Ashmore, R.M. Noyes, L. Valentine, N. Miller, G.C. Bond, J.S. Rowlinson, F.S. Dainton, General and physical chemistry. Ann. Rep. Progress Chem. 52, 7–92 (1955)
V.M. Goldschmidt, T. Barth, G. Lunde, W.H. Zachariasen, Geochemical distribution law of the elements. VII. Summary of the chemistry of crystals. Skr. Nor. Vidensk. Akad 1, 1–117 (1926)
V.M. Goldschmidt, Crystal structure and chemical correlation. Ber. Deut. Chem. Ges. 60, 1263–1296 (1927)
V.M. Goldschmidt, The distribution of the chemical elements 1. Nature 124, 15–17 (1929)
L.H. Ahrens, The significance of the chemical bond for controlling the geochemical distribution of the elements-part 1. Phys. Chem. Earth 5, 1–54 (1964)
D. Robert Hay, P.D. Parikh, Valence bond interpretation of elastic anisotropy in BCC transition metals. Phil. Mag. 20(166), 753–758 (1969)
W.H. Zachariasen, A set of empirical crystal radii for ions with inert gas configuration. Zeitschrift für Kristallographie-Cryst. Mater. 80(1–6), 137–153 (1931)
W.H. Zachariasen, Crystal radii of the heavy elements. Phys. Rev. 73(9), 1104 (1948)
W.L. Bragg, XVIII The arrangement of atoms in crystals. Lond. Edinburgh Dublin Philos. Mag. J. Sci. 40(236), 169–189 (1920)
J.K. Nagle, Atomic polarizability and electronegativity. J. Am. Chem. Soc. 112(12), 4741–4747 (1990)
J.C. Slater, Atomic radii in crystals. J. Chem. Phys. 41(10), 3199–3204 (1964)
J.C. Slater, Quantum Theory of Molecules and Solids: Symmetry and Energy Bands in Crystals, vol. 2 (McGraw-Hill, New York, 1963).
D.R. Hartree, The calculation of atomic structures (1957).
E. Clementi, D.L. Raimondi, Atomic screening constants from SCF functions. J. Chem. Phys. 38(11), 2686–2689 (1963)
D. Liberman, J.T. Waber, D.T. Cromer, Self-consistent-field Dirac-Slater wave Functions for atoms and ions. I. Comparison with previous calculations. Phys. Rev. 137(1), A27 (1965)
C. Froese, Hartree—Fock parameters for the atoms helium to radon. J. Chem. Phys. 45(5), 1417–1420 (1966)
E. Clementi, D.L. Raimondi, W.P. Reinhardt, Atomic screening constants from SCF functions. II. Atoms with 37 to 86 electrons. J. Chem. Phys. 47(4), 1300–1307 (1967)
C. Fisk, S. Fraga, Atomic Radii. In Anales de fisica . Facultad de fisica quimica ciudad univ, 3 Madrid, vol. 65, ( Real Soc Espan Quimica, Spain), p. 135 (1969).
A. C. Larson, J. T. Waber. Self-consistent field Hartree Calculations for Atoms and Ions (No. LA- 4297). Los Alamos Scientific Lab., N. Mex (1969).
C.F. Fischer, Average-energy-of-configuration Hartree-Fock results for the atoms helium to radon charlotte Froese Fischer. Atom. Data Nucl. Data Tabl. 12, 301–399 (1972)
C.W. Kammeyer, D.R. Whitman, Quantum mechanical calculation of molecular radii. I. Hydrides of elements of periodic groups IV through VII. J. Chem. Phys. 56(9), 4419–4421 (1972)
S. Fraga, J. Karwowski, K.M.S. Saxena, Hartree-Fock values of coupling constants, polarizabilities, susceptibilities, and radii for the neutral atoms, helium to nobelium. At. Data Nucl. Data Tables 12(5), 467–477 (1973)
C.F. Fischer. Atom. Data 4 (1972) 301. Atom. Data Nucl. Data, 12, 87 (1973).
J.P. Desclaux, Atomic Data Nucl. Data Tables 12(4), 311 (1973)
R.J. Boyd, The relative sizes of atoms. J. Phys. B At. Mol. Phys. 10(12), 2283 (1977)
B.M. Deb, R. Singh, N. Sukumar, A universal density criterion for correlating the radii and other properties of atoms and ions. J. Mol. Struct. (Thoechem) 259, 121–139 (1992)
D. Ghosh, R. Biswas, Theoretical calculation of absolute radii of atoms and ions. Part 1. The atomic radii. Int. J. Mol. Sci. 3(2), 87–113 (2002)
M.V. Putz, N. Russo, E. Sicilia, Atomic radii scale and related size properties from density functional electronegativity formulation. J. Phys. Chem. A 107(28), 5461–5465 (2003)
P. Pyykko, S. Riedel, M. Patzschke, Chem. Eur. J.11: 3511 (2005).
D.C. Ghosh, R. Biswas, T. Chakraborty, N. Islam, S.K. Rajak, J. Mol. Struct. (Theochem) 865, 60 (2008)
T. Chakraborty, K. Gazi, D.C. Ghosh, Computation of the atomic radii through the conjoint action of the effective nuclear charge and the ionisation energy. Mol. Phys. 108(16), 2081–2092 (2010)
J.C. Slater, Atomic shielding constants. Phys. Rev. 36(1), 57 (1930)
R.T. Sanderson, The covalent radius of radon and the electronegativities of gold, mercury, thallium, lead, and bismuth. J. Inorg. Nucl. Chem. 7, 288 (1958)
A. Bondi, Vander Waals volumes and radii. J. Phys. Chem. 68(3), 441–451 (1964)
W.H. Zachariasen, A set of empirical crystal radii for ions with inert gas configuration. Zeitschriftfür Kristallographie-Crystall. Mater. 80(1–6), 137–153 (1931)
P.W. Atkins, R.S. Friedman, Molecular Quantum Mechanics, 3rd edn. (Oxford University Press, Oxford, 1997).
T. Brinck, J.S. Murray, P. Politzer, J. Chem. Phys. 98, 4305 (1993)
S. Hati, D. Dutta, J. Phys. Chem. 98, 10451 (1994)
E.M. Purcell, Berkley Physics Course, TMH Edition, Vol. 2, (Tata McGraw-Hill Publishing Company, Bombay) (1963).
O. Roberto, On weak interactions as short-distance manifestations of gravity. Mod. Phys. Lett. A 28, 1350022 (2013)
U.V.S. Seshavatharam, S. Lakshminarayana, Role of four gravitational constants in nuclear structure. Mapana J. Sci. 18(1), 21–46 (2019)
R.S. Mulliken, Electronic population analysis on LCAO–MO molecular wave functions I. J. Chem. Phys. 23(10), 1833–1840 (1955)
R.G. Parr, R.G. Pearson, Absolute hardness: companion parameter to absolute Electronegativity. J. Am. Chem. Soc. 105(26), 7512–7516 (1983)
G. Klopman, Chemical reactivity and the concept of charge-and frontier-controlled Reactions. J. Am. Chem. Soc. 90(2), 223–234 (1968)
D.C. Ghosh, T. Chakraborty, Gordy’s electrostatic scale of electronegativity revisited. J. Mol. Struct. (Thoechem) 906(1–3), 87–93 (2009)
P.W. Ayers, The physical basis of the hard/soft acid/base principle. Faraday Discuss. 135, 161–190 (2007)
P. Geerlings, F. De Proft, W. Langenaeker, Conceptual density functional theory. Chem. Rev. 103(5), 1793–1874 (2003)
R.G. Parr, R.G. Pearson, J. Am. Chem. Soc. 105, 7512 (1983)
R.G. Pearson, J. Chem. Educ. 64, 561 (1987)
R.G. Parr, W. Yang, Density Functional Theory of Atoms and Molecules (Oxford University Press, New York, 1989).
R.P. Feynman, R.B. Leighton, M. Sands, The Feynman Lecture on Physics, Addison– Wesley: Mass., Vol. II., (1964).
P.B. Janardhan, B. Sivasankar, A Text-Book of Inorganic Chemistry (Oxford and IBH, New Delhi, 1978).
D.C. Ghosh, N. Islam, Semiempirical evaluation of the global hardness of the atoms of 103 elements of the periodic table using the most probable radii as their size descriptors. Int. J. Quantum Chem. 110(6), 1206–1213 (2010)
D.C. Ghosh, R. Biswas, Theoretical calculation of absolute radii of atoms and ions. Part 1. The atomic radii. Int. J. Mol. Sci 3(2), 87–113 (2002)
R.P. Shalini, T. Chakraborty, Theoretical Computation of Periodic Descriptors Invoking Periodic Properties, in Chemical Science and Engineering Technology. ed. by C.A. Suresh, C. Tanmoy (Apple Academic Press, Waretown, 2019), pp. 31–40
Acknowledgements
Dr. Tanmoy Chakraborty is thankful to Sharda University for providing computational resources and research facility.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shalini Chaudhary, Chaudhary, A., Rajak, S.K. et al. Theoretical computation of normalised radii, density and global hardness as a function of orbital exponent. J Math Chem 59, 1014–1028 (2021). https://doi.org/10.1007/s10910-021-01224-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10910-021-01224-8