Abstract
The utilization of arginase for catalyzing the synthesis of L-arginine into L-ornithine has proven to be an effective industrial production process. The poor stability of arginase hampers its synthesis efficiency. Organic-inorganic hybrid nanoflowers serve as an effective strategy for enzyme immobilization. However, the precipitation of such immobilized enzymes is prone to dissolution and destruction in amino acid solutions, limiting their applicability. This paper systematically investigates the solubility of organic-inorganic hybrid nanoflowers formed with common metal ions in typical amino acid solutions. Additionally, a novel approach involving the preparation of calcium phosphate hybrid nanoflowers using crude arginase is developed. The findings confirm that the immobilized arginase exhibits superior stability and a broader application range. In the reaction system where L-arginine is utilized as a substrate for synthesizing ornithine, the immobilized arginase demonstrates higher substrate conversion rates and ornithine concentrations compared to free arginase crude extract. This approach holds the potential for industrial applications due to its improved performance.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
L-Ornithine has been implicated in various diseases such as hyperkalemia, hyperammonemia, and cancer [1]. Moreover, it exhibits hepatoprotective effects [2], aids in treating acute liver injury [3], enhances plant drought resistance [4], promotes wound healing [5], and contributes to the treatment of chronic periodontitis [6]. Arginase plays a crucial role in catalyzing the conversion of L-arginine to L-ornithine and urea [7, 8]. Consequently, L-ornithine is commonly produced from L-arginine through arginase. However, the instability of arginase necessitates a substantial enzyme quantity during production process, leading to higher production costs.
Enzyme immobilization has been employed as a strategy to enhance enzyme stability, extend working time, and reduce production costs. Among traditional methods, covalent immobilization involves forming covalent bonds between the enzyme and carrier. While this approach significantly improves enzyme stability and recyclability, it may compromise the enzyme structure, leading to a loss of enzymatic activity [9, 10], making it suitable for some limited sets of enzymes. Encapsulation immobilization strategy utilizes materials like carrageenan or calcium alginate to encapsulate enzymes, protecting with minimal damage. However, this method can hinder substrate-enzyme interactions, reducing overall reaction efficiency [11, 12].
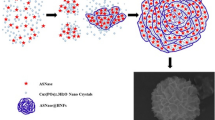
A novel approach involves protein-inorganic hybrid nanoflowers, where a flower-shaped nanostructure is formed by coordinating enzymes with metal ions and phosphates. This method minimizes damage to enzyme structures and greatly increases the specific surface area, facilitating enhanced substrate-enzyme contact. Numerous cases have demonstrated that the formation of nanoflowers significantly improves enzyme stability and catalytic activity [13,14,15]. There are several precedents for immobilizing various biological molecules, including enzymes [16, 17], amino acids [18, 19], nucleic acids [20], and biological extracts [21], in different phosphate metal salts. The process of enzyme purification is usually inefficient, time-consuming, and labor-intensive. Consequently, in practical applications, it is frequently observed that the crude extract from Escherichia coli cells overexpressing the enzyme is directly immobilized. This approach typically enhances the efficiency and exerts minimal influence on the nanoflower structure formation and the enzymatic catalytic activity. Chen et al. initially synthesized nanoflowers utilizing the purified form of N-acyl homoserine lactonase and subsequently investigated their activity and stability. In subsequent practical applications, they opted to fabricate nanoflowers using the extract derived from Escherichia coli cells that express lactonase. This method yielded results that were similarly commendable. [14]. Due to its simple preparation method and environmentally friendly nature, this immobilization technique holds significant promise for widespread application in the industrial sector.
According to the coordination theory proposed by Dube et al., the synthesis of organic-inorganic hybrid nanoflowers is orchestrated through a series of coordinated interactions. Initially, metal ions engage in a reaction with phosphate, culminating in the formation of nascent phosphate metal precipitate particles. These particles then undergo a coordination process with the amino acids that are integral to protein structures, leading to the emergence of primary crystals. As the process progresses, these primary crystals aggregate with additional proteins, forming more substantial polymer precipitates. This assembly ultimately results in the creation of insoluble, flower-like nanostructures. These structures are hybrid entities that amalgamate inorganic phosphate precipitates with organic protein constituents, thereby facilitating the immobilization of enzymes [22]. The presence of small molecule compounds with potent coordination capabilities, such as ethylenediaminetetraacetic acid (EDTA), can disrupt the nanoflower architecture. These molecules can competitively form complexes with the metal ions embedded within the nanoflowers. This interaction prompts the disassembly and dissolution of the nanoflower structure, effectively reverting the immobilized enzymes back to their free state [23]. From a theoretical standpoint, the carboxyl and amino groups present within amino acids are equipped to form coordination bonds with metal ions. This suggests that in solutions with elevated amino acid concentrations, these amino acids could potentially outcompete for the metal ions present in the organic-inorganic hybrid nanoflowers. Such competition could lead to the disassembly, dissolution, and reactivation of the immobilized enzyme precipitates. In light of this theoretical framework, it is hypothesized that the immobilization of enzymes via the organic-inorganic hybrid nanoflower strategy may not be optimal for reaction systems that contain significant concentrations of amino acid compounds. An example of such a system would be the reaction pathway for the synthesis of ornithine from arginine, where the presence of high amino acid concentrations could interfere with the stability and effectiveness of the immobilized enzymes.
To verify the above hypothesis, this study first investigated the solubility of hybrid nanoflowers formed by different metal ions in the presence of 20 kinds of natural amino acids. This exploration provides valuable insights and serves as a reference for utilizing hybrid nanoflowers in constructing immobilized enzymes for reaction systems containing amino acids in future applications. Based on this, a method for immobilizing nanoflower-like arginase using calcium phosphate was developed. The nanoflowers underwent testing to assess their morphology, activity, and stability, leading to the establishment of a viable process for producing heat-resistant nanoflowers with efficient arginine hydrolysis capabilities. This process was proved to be effective in the enzymatic synthesis of L-ornithine.
2 Materials and Methods
2.1 Chemicals and Reagents
The salts (ZnCl2, CaCl2, MnCl2, CoCl2, NiSO4, CuSO4) utilized in the preparation of nanoflowers and the salts (Na2HPO4, KH2PO4, KCl, NaCl, Tris, Glycine, NaOH) employed for the formulation of buffer solutions, along with twenty natural amino acids and other chemical reagents (kanamycin sulfate, glycerol, indole-3-ketone, dithiothreitol, acetic acid, and phosphate), were purchased from Sangon Biotech (Shanghai, China). The yeast extract and peptone for TB medium were sourced from OXOID (UK).
2.2 Preparation of Metal Phosphate Precipitation and Cell Extract@Metal Phosphate Hybrid Nanoflowers
In 1 mL of 10 mmol/L PBS solution, different metal ions (Ni2+, Co2+, Mn2+, Zn2+, Cu2+ or Ca2+) solutions were added to achieve a final concentration of 10 mmol/L. The mixture was then incubated at 25 °C for approximately 10 h. The resulting precipitate was collected by centrifugation, washed with deionized water, and prepared into the corresponding metal phosphate precipitate.
Escherichia coli cell obtained from the culture was diluted in 10 mmol/L PBS buffer to 10%, and high-pressure homogenization was employed to disrupt the cells. After centrifugation at 12,000 g for 30 min, the supernatant was collected to obtain the cell extract. The cell extract was then diluted with 10 mmol/L PBS buffer to a total protein content of 0.28 mg/mL. To prepare cell extract@metal phosphate hybrid nanoflowers, 1000 µL of cell extract solution was mixed with different metal ions (Ni2+, Co2+, Mn2+, Zn2+, Cu2+ or Ca2+) solutions to achieve a final concentration of 10 mmol/L. The mixture was incubated at 25 °C for approximately 10 h, followed by centrifugation to collect the precipitate. After washing with deionized water, the corresponding cell extract@metal phosphate hybrid nanoflowers were obtained.
2.3 Impact of Different Amino Acids on the Solubility of Metal Phosphate Precipitates and Cell Extract@Metal Phosphate Hybrid Nanoflowers
Amino acid solutions were prepared with a final concentration of 100 mmol/L, and the pH was adjusted to pH = 7.4 using HCl or NaOH. It is noted that L-tyrosine did not completely dissolve in the solution at pH = 7.4. Cell extract@metal phosphate hybrid nanoflowers (CE@M(II)-hNFs) or the respective metal phosphate suspensions were mixed with 1 mL of amino acid storage solution, and the mixture was stood for 24 h to observe the dissolution of nanoflowers and metal phosphates in the amino acid solution. The amino acids used included L-alanine, L-arginine, L-asparagine, L-aspartic acid, L-cysteine, L-glutamine, L-glutamic acid, L-glycine, L-histidine, L-isoleucine, L-leucine, L-lysine, L-methionine, L-phenylalanine, L-proline, L-serine, L-threonine, L-tryptophan, L-tyrosine and L-valine.
2.4 Preparation of Arginase
The arginase employed in this study originated from Brevibacillus brevis strain 47, Uniport ID: C0ZIM2. The encoding gene for arginase was cloned into the pET28a vector and expressed in E. coli strain BL21(DE3). The E. coli cell expressing arginase was cultured in TB medium containing 50 mg/L kanamycin at 37 °C with agitation at 200 rpm until OD600 reached 0.6. Induction of arginase expression was achieved by adding IPTG to a final concentration of 0.2 mmol/L, and cultivation was continued at 37 °C for 5 h.
Arginase purification was conducted from crude cell extracts of the E. coli cell expressing arginase. Initially, the fermented broth was centrifuged at 4000 g for 20 min to collect the bacterial cells from the precipitate. Cell disruption was performed at 4 °C, and the supernatant containing cell extracts was recovered after centrifugation at 8000 g for 30 min. Subsequently, Ni2+-NTA affinity chromatography (GE Healthcare, Uppsala, Sweden) was employed for arginase purification according to the manufacturer’s instructions. Finally, gel filtration chromatography using SephadexTM G-25 M (GE Healthcare, Uppsala, Sweden) was performed to eliminate salt ions from the protein sample. The obtained arginase was stored in a storage solution (100 mmol/L NaCl, 1 mmol/L DTT, 500 g/L glycerol, and 50 mmol/L PB). The concentration of arginase was determined using the modified Bradford protein assay kit (Sangon Biotech, China).
2.5 Determination of Arginase Activity
The method for arginase activity determination was adapted from Francis P. Chinard [24] and Xiaoli Zhang et al. [25] with some modifications. Based on this foundation, a method for measuring arginase activity was developed: Prepare an 800 µL reaction system with glycine-NaOH buffer (pH = 10, 50 mmol/L), The system includes 5 g/L L-arginine, 0.25 µg/mL free or immobilized arginase, and 5 mmol/L Ni2+. Shake the reaction system at 70 °C for 10 min and then add 800 µL acetic acid to terminate the reaction. Centrifuge the mixture at 5000 g for 3 min, Extract 1000 µL of the supernatant into a 2 mL microcentrifuge tube. Add 500 µL of acidified ninhydrin (25 mg/mL) to the microcentrifuge tube and incubate at 95 °C for 1 h. Measure the absorbance of the solution at 515 nanometers. Utilize samples without arginase as a reference. Generate a standard curve using different concentrations of L-arginine solutions. Define one unit of arginase activity as the enzyme quantity required to produce 1µmol of L-ornithine per minute.
2.6 Preparation and Evaluation of Arginase@Hybrid Nanoflowers
Arginase@hybrid nanoflowers were prepared based on the procedure described by Ge et al., with certain modifications [23]. Metal salt solutions (ZnCl2, CaCl2, MnCl2, CoCl2, NiSO4, CuSO4) were prepared at a concentration of 100 mmol/L. Purified arginase was diluted to a final concentration of 0.25 mg/mL in 10 mmol/L PBS buffer. Hybrid nanoflowers were formed by incubating 100 µL of the metal salt solution with 1000 µL of the arginase solution at 25 °C for 9 h. The resulting precipitates were collected by centrifugation at 3000 g for 3 min and washed with deionized water 2–3 times. The obtained arginase-inorganic hybrid nanoflowers were stored at 25 °C for subsequent use.
The concentration of non-immobilized arginase in the supernatant was determined using the modified Bradford protein assay kit. The amount of immobilized arginase was calculated by subtracting the non-immobilized arginase from the total arginase. Encapsulation efficiency was calculated as the ratio of immobilized arginase to the total arginase. Arginase activity was measured before and after immobilization.
2.7 Optimization of Conditions for the Preparation of Arginase@Calcium Phosphate Hybrid Nanoflowers
Building upon the aforementioned preparation process, an investigation into the optimization of conditions for the synthesis of arginase@calcium phosphate hybrid nanoflowers (arginase@Ca-hNFs) was conducted. Various factors, including the types of metal ions (Ni2+, Co2+, Mn2+, Zn2+, Cu2+ and Ca2+), enzyme concentration (0.05–0.40 mg/mL), incubation time (1–24 h), incubation temperature (4, 25, and 37 °C), and centrifugation speed (3000–13,000 g), were systematically examined to assess their impact on the encapsulation efficiency and catalytic activity of the arginase hybrid nanoflowers. The goal was to determine the optimal conditions for the preparation process.
2.8 Enzymatic Properties of Immobilized Arginase
The catalytic activity of free and immobilized arginase was investigated in the presence of various divalent metal ions (Ni2+, Co2+, Mn2+, Zn2+, Cu2+ and Ca2+), with the activity of free arginase without added metal ions serving as a control. The impact of Ni2+ concentration in the range of 0–25 mmol/L on the activities of both free and immobilized arginase was determined. Additionally, the effects of temperature (30–80 °C) and pH (7–9, 50 mmol/L Tris-HCl buffer and 10–12, 50 mmol/L glycine-NaOH buffer) on the activities of free and immobilized arginase were investigated.
Furthermore, free or immobilized arginase was incubated in phosphate buffer (pH 7.4) at different temperatures (4–60 °C) for 24 h. The residual activity was measured under standard conditions to assess the impact of temperature on the stability of free and immobilized arginase. The stability of free and immobilized arginase was also evaluated by incubating the enzymes in buffers at different pH (50 mmol/L Tris-HCl, pH 7–9 and 50 mmol/L glycine-NaOH, pH 10–12) at 30 °C for 24 h, followed by measuring the residual activity under standard conditions.
Additionally, free or immobilized arginase was subjected to prolonged incubation (1–5 days) under the most stable temperature and pH conditions. Residual activity was measured under standard conditions to assess the long-term stability of free and immobilized arginase.
2.9 Characterization of Arginase@Calcium Phosphate Hybrid Nanoflowers
The morphology of calcium phosphate and arginase@Ca-hNFs was observed using the ZEISS Gemini 500 scanning electron microscope (SEM). Additionally, X-ray diffraction (XRD) analysis was conducted using the Thermo ARL ACINTAG X’TRA.
2.10 Optimization of the Immobilization Process for Crude Arginase
The bacterial cells expressing arginase were diluted in 10 mmol/L PBS buffer to a concentration of 100 g/L. Cell disruption was achieved using a high-pressure homogenizer, and after centrifugation at 12,000 g for 30 min, the supernatant containing crude arginase was collected. The crude arginase was then diluted to 0.28 mg/mL (total protein concentration) in 10 mmol/L PBS buffer.
The immobilization of the enzyme followed an optimized process. In a 40 mL volume of the crude enzyme solution, 4 mL of CaCl2 solution (100 mmol/L) was added, and the mixture was incubated at 25 °C for 11 h. After centrifugation at 3000 g for 3 min, the precipitate was collected. The obtained immobilized crude enzyme was washed with deionized water 2–3 times and stored at 25 °C for further use.
Building on this foundation, the impact of various parameters, including total protein concentration (0.05–0.40 mg/mL), incubation time, and nickel ion concentration, on the catalytic activity of crude arginase@Ca-hNFs was systematically investigated to determine the optimal preparation conditions.
2.11 Biocatalysis of L-Arginine to L-ornithine Using Immobilized Crude Arginase
A reaction solution of 100 mL was prepared, comprising 200 g/L L-arginine, 0.1 mg/mL of free or a considerable amount of immobilized crude arginase, and 40 mmol/L Ni2+. The reaction was carried out in a stirred water bath at pH 9.0 and 40 °C for 20 h. Samples were collected at 2-hour intervals, and the concentration of L-ornithine was determined.
3 Results
3.1 Influence of Amino Acids on the Solubility of Metal Phosphates and Cell Extract@Metal Phosphate Hybrid Nanoflowers
To investigate the influence of common amino acids on the solubility of hybrid nanoflowers, we prepared phosphate precipitates of nickel, cobalt, manganese, copper, zinc, and calcium, as well as the corresponding cell extract hybrid nanoflowers (CE@hNFs). CE@hNFs were then mixed with individual amino acids. As illustrated in Fig. 1, the overall solubility trends of amino acids in both phosphate precipitates and corresponding CE@hNFs were consistent. Notably, for zinc, cobalt, and copper, the stability of the CE@hNFs precipitates was slightly higher than that of the sole phosphate precipitates.
The impact of amino acids was most significant for nickel phosphate precipitates and the corresponding CE@hNFs. In the presence of most amino acids, except for Tyr, the precipitates completely dissolved. Partial or complete dissolution was observed for cobalt and copper phosphates after mixing with the majority of amino acids. Zinc phosphate precipitates showed partial solubility in amino acid solutions, and the CE@Zn-hNFs precipitates remained stable in solutions containing most amino acids, except for cysteine and histidine. Calcium and manganese phosphate precipitates, along with their cell extract hybrid counterparts, demonstrated stability in solutions with all tested amino acids.
3.2 Preparation and Evaluation of Arginase@Metal Phosphate Hybrid Nanoflowers
Under the conditions specified in the Materials and Methods, calcium, manganese, copper, zinc, cobalt, and nickel metal ions all formed precipitates with purified arginase in phosphate buffer. However, during the subsequent assessment of nanoflower activity, consistent with the earlier findings, the nanoflower precipitates of nickel, zinc, and cobalt rapidly dissolved in arginine solution at pH 10. In contrast, the arginase@calcium phosphate nanoflower (arginase@Ca-hNFs) precipitate remained stable, but the arginase@manganese phosphate nanoflower (arginase@Mn-hNFs) precipitate also dissolved in arginine solution at pH 10. This suggests that the solubility of phosphate precipitates in amino acid solutions is not only influenced by the type of amino acids but also is pH-dependent. Detailed results are presented in Fig. 2. These findings highlight that only the arginase@Mn-hNFs were able to maintain their insoluble and immobilized state in a high concentration of arginine solution at pH 10 and may find application in catalytic reactions involving arginine. In contrast, nanoflowers formed by arginase with several other metal ions, such as ZnCl2, CaCl2, MnCl2, CoCl2, NiSO4, and CuSO4, dissolved in arginine-rich solutions, reverting back to the free enzyme state. This finding suggests that the immobilized arginases formed with these metal ions are not suitable for catalyzing the decarboxylation of arginine to produce L-ornithine. Consequently, based on the aforementioned experimental outcomes, our subsequent research focused solely on elucidating the structural characteristics and enzymatic properties of arginase@Ca-hNFs, while nanoflowers formed with arginase and other metal ions were not further examined or presented in this study.
3.3 Optimization of Arginase@Calcium Phosphate Hybrid Nanoflower Preparation
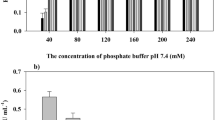
Under constant metal phosphate concentrations, enzyme concentration affects the morphology, structure, and properties of formed nanoflowers. We investigated the influence of arginase concentrations ranging from 0.05 to 0.40 mg/mL under calcium ion conditions of 10 mmol/L, as shown in Fig. 3a. At an arginase concentration of 0.25 mg/mL, complete embedding into nanoflowers with stable activity was achieved, while higher concentrations led to decreased unit activity and embedding efficiency. Considering potential mechanical strength reduction at lower organic component concentrations, subsequent nanoflower preparation was set at 0.25 mg/mL.
The formation time of nanoflowers, involving calcium phosphate precipitation and self-assembly processes, affects their properties. We assessed the embedding efficiency and catalytic activity of nanoflowers formed within a time range of 1–24 h. As shown in Fig. 3b, within the 1–7 h interval, enzyme activity and embedding efficiency increased with preparation time. Between 9 and 24 h, both enzyme activity and embedding efficiency remained relatively stable. Therefore, the subsequent preparation time was fixed at 9 h. Temperature significantly influences enzyme stability and calcium phosphate solubility, thereby affecting the final nanoflower properties. We investigated the properties of nanoflowers formed at 4, 25, and 37 °C. As shown in Fig. 3b, higher temperatures accelerated nanoflower formation, increasing embedding efficiency. Considering energy consumption and arginase stability in practical applications, the subsequent nanoflower preparation conditions were set at 25 °C with a 9-hour incubation period.
After nanoflower formation, separation from the solution system is required. Different separation methods and conditions exert varying pressures on nanoflowers, potentially impacting their structure, morphology, and catalytic properties. We examined the influence of different centrifugation speeds on nanoflower activity, as shown in Fig. 3c. Centrifugal forces below 5000 g did not affect nanoflower activity. However, as the centrifugal force increased, nanoflower activity noticeably decreased. When centrifugal force was further increased to 13,000 g, activity was preserved at less than 60%. We hypothesize that excessive centrifugal force leads to nanoflower structural collapse, resulting in decreased activity. To ensure complete nanoflower collection and preserve their structure, the chosen nanoflower collection condition was 3000 g for 3 min.
In summary, the selected preparation system involved adding 100 µL of 100 mmol/L calcium chloride to 1000 µL of 0.25 mg/mL purified arginase protein in 10 mmol/L PBS buffer, followed by incubation at 25 °C for 9 h. The supernatant was then separated by centrifugation at 3000 g for 3 min, collecting the nanoflower precipitate.
3.4 Characterization of Arginase@Calcium Phosphate Hybrid Nanoflowers
The successful synthesis of arginase-based organic-inorganic hybrid nanoflowers has been verified through Scanning Electron Microscopy (SEM), Energy Dispersive Spectroscopy (EDS), Fourier Transform Infrared Spectroscopy (FTIR), and X-ray Diffraction (XRD). These techniques further elucidate the spatial structure, elemental composition, chemical structure, and the mode of interaction between the organic and inorganic components.
SEM results revealed that, in contrast to the simple flake-like structure of phosphate crystals (Fig. 4a), arginase organic-inorganic hybrid nanoflowers can form a flower-like structure with a diameter of approximately 0.5 μm (Fig. 4b). Arginase played a role akin to “glue” during the formation of the organic-inorganic hybrid nanoflowers, enabling the flake-like phosphate “petals” to form a flower-like structure through stacking.
EDS analysis of the arginase organic-inorganic hybrid nanoflowers demonstrated that the main chemical elements are O, P, and Ca, with trace amounts of C, Cl, and Na (Fig. 4c). This confirms that the hybrid nanoflowers are composed of both organic and inorganic components.
To ascertain the chemical structure of the inorganic component, XRD analysis was performed on the nanoflowers and phosphate crystals. XRD results showed that the peaks of both the phosphate and the arginase organic-inorganic hybrid nanoflowers are essentially consistent with the standard pattern of hydrated Ca3(PO4)2 (PDF 18–0303), thereby confirming that the inorganic component of the nanoflowers is hydrated Ca3(PO4)2 (Fig. 4d).
Finally, FTIR was utilized to further analyze the organic component of the arginase organic-inorganic hybrid nanoflowers and the interaction between the organic and inorganic components. The FTIR spectra of arginase, Ca3(PO4)2, and the arginase@Ca-hNFs powder samples are shown in Fig. 4e. A broad O-H stretching vibration peak at 3385 cm− 1 was observed for both Ca3(PO4)2 and arginase@Ca-hNFs, indicating the presence of hydrated Ca3(PO4)2. The arginase@Ca-hNFs sample exhibited a strong P-O stretching vibration absorption peak at 1023 cm− 1, characteristic of Ca3(PO4)2. Additionally, the arginase@Ca-hNFs sample showed a protein amide I band at 1653 cm− 1 (1640–1660 cm− 1, C = O stretching vibration), an amide II band at 1546 cm− 1 (1600 –1500 cm− 1, N-H stretching vibration), and in-plane bending vibrations of C-H and O-H bonds at 1408 cm− 1, indicating the organic component as arginase. Compared to the FTIR spectrum of the arginase sample, no significant peak shifts were observed in the amide I and amide II bands of the arginase@Ca-hNFs FTIR spectrum, and no new absorption peaks appeared, suggesting that the arginase@Ca-hNFs is formed by non-covalent bonding between arginase and Ca3(PO4)2, with no covalent bond formation during the self-assembly process.
Structural characterization of arginase@Ca-hNFs: a Scanning electron microscope (SEM) images of calcium phosphate (wide-angle, left; detailed view, right); b Scanning electron microscope (SEM) images of arginase@Ca-hNFs (wide-angle, left; detailed view, right); c X-ray diffraction (XRD) spectrum of arginase@Ca-hNFs and calcium phosphate. d FTIR of Free arginase, Ca3(PO3)2 and arginase@Ca-hNFs, e EDS of arginase@Ca-hNFs
3.5 Enzymatic Properties of Free and Immobilized Arginase
We selected arginine as a model substrate to determine the enzymatic properties of free and immobilized arginase under various experimental parameters such as metal ion type and concentration, pH, temperature, storage pH, storage temperature, and storage time.
As shown in Fig. 5a, several common divalent metal ions exhibited similar effects on both free and immobilized arginase. Ni2+, Co2+, and Mn2+ demonstrated strong activation effects, with Ni2+ showing an activation up to 50 times. Zn2+ and Cu2+ exhibited significant inhibition on arginase, while Mg2+ and Ca2+ had minimal impact.
Next, we investigated the effect of nickel ion concentration on the catalytic activity of free and immobilized arginase, as illustrated in Fig. 5b. Within the concentration range of 5–10 mmol/L, nickel ions activated the catalysis of arginase. Excessively low or high nickel ion concentrations weakened the activation effect on the enzyme.
As depicted in Fig. 5c, both free and immobilized arginase exhibited optimal activity at 70 °C. Activity sharply declined at temperatures above 80 °C, reaching complete loss around 90 °C. In the range of 30–60 °C, immobilized arginase showed higher activity than free arginase, maintaining over 50% of the highest activity in the 40–80 °C range.
The enzymatic activity trend for free and immobilized arginase was similar across the pH range of 7–12, as shown in Fig. 5d. The highest activity was observed at pH 10 for both forms, with immobilized arginase retaining 60% of the highest activity in the pH 8–12 range, slightly better than free enzyme.
After 24 h of storage under different conditions, we measured the relative activity of free and immobilized arginase to assess the impact of storage pH, storage temperature, and storage time on stability. Figure 5e reveals that after incubation at 60 °C for 24 h, the activity of both free and immobilized arginase was nearly completely lost. However, the stability of free arginase significantly decreased above 40 °C, while immobilized arginase, even after incubation at 50 °C for 24 h, retained over 60% activity. This indicates that immobilized arginase has better temperature stability and longer endurance.
Figure 5f illustrates that the stability trend of free and immobilized arginase under different pH values is similar, with immobilized arginase showing slightly better stability than free arginase.
In summary, despite the similarities in optimum temperature, optimum pH, and activity between free and immobilized arginase, immobilized arginase exhibits a broader range of temperature and pH adaptability compared to its free counterpart. This suggests that immobilized arginase may hold superior industrial production potential over free arginase.
Enzymatic characteristics of free and immobilized arginase: a Effect of metal ion types on the activity of free and immobilized arginase; b Effect of nickel ion concentration on the activity of free and immobilized arginase; c Effect of temperature on the activity of free and immobilized arginase; d Influence of pH buffer (pH 7–9 (Tris-HCl), pH 10–12 (glycine-NaOH)) on the activity of free and immobilized arginase; e Temperature stability of free and immobilized arginase activity; f pH stability of free and immobilized arginase
3.6 Preparation and Evaluation of Cell Extract@Calcium Phosphate Hybrid Nanoflowers
The process of preparing nanoflowers using purified arginase is intricate, time-consuming, and costly. To simplify the manufacturing process and reduce costs in practical applications, we opted to directly utilize E. coli cell extract with high arginase expression for nanoflower preparation. We investigated the impact of different preparation conditions on the arginase activity and encapsulation efficiency of the cell extract.
As shown in Fig. 6a, when the total protein concentration exceeded 0.28 mg/mL, the activity of nanoflowers gradually decreased with the increase in protein concentration, resembling the characteristics observed with nanoflowers prepared from purified enzymes. We further studied the effect of incubation time on the activity of nanoflowers obtained from crude extract, as depicted in Fig. 6b. With prolonged incubation time, the activity of nanoflowers gradually increased and reached a steady state after 11 h. Therefore, we selected conditions involving the addition of 100 mL calcium chloride solution (100 mmol/L) to 1 L PBS buffer containing 0.28 mg/mL cell extract protein, followed by an 11-hour incubation at 25 °C. The resulting precipitate was collected to prepare cell extract@calcium phosphate hybrid nanoflowers (CE@Ca-hNFs).
Additionally, due to significant differences in enzyme quantity and arginine concentration in the actual synthesis of L-ornithine reaction systems, we investigated the influence of nickel ion concentrations on the catalytic abilities of both cell extract and equivalent nanoflowers in a system containing 0.1 mg/mL crude protein, along with 200 g/L arginine, at pH 9 and 40 °C, as illustrated in Fig. 6c. The trend of nickel ions affecting arginase activity was similar to that observed with purified arginase, and an optimal nickel ion concentration of 40 mmol/L was chosen for the ornithine synthesis reaction system.
Preparation and reaction condition optimization of crude arginase@Ca-hNFs: a Effect of total protein concentration on activity and encapsulation rate; b Effect of incubation time on activity and encapsulation rate; c Effect of nickel ion concentration on activity under conditions of 40 °C, pH 9 (Tris-HCl, 50 mmol/L)
3.7 Biocatalytic Transformation Using Crude Arginase and Crude Arginase@Ca-hNFs
Considering the extended duration of the L-ornithine synthesis reaction and the enzymatic activity and stability within the reaction system, we selected reaction conditions of 40 °C and pH 9.0. The reaction system for the conversion to produce L-ornithine contained 200 g/L L-arginine, 0.1 mg/mL of cell extract or an equivalent concentration of nanoflowers, with a final Ni2+ concentration of 40 mmol/L.
As shown in Fig. 7, the CE@Ca-hNFs demonstrated superior activity from the onset of the reaction. Furthermore, after a reaction period exceeding 20 h, the CE@Ca-hNFs converted over 99.02% of the substrate, resulting in an L-ornithine concentration exceeding 150.28 g/L. In contrast, the free crude arginine converted only approximately 71.13% of the substrate, resulting in an L-ornithine final concentration of approximately 107.95 g/L. The CE@Ca-hNFs exhibited higher yields compared to crude arginine. This suggests a significant advantage of crude arginine nanoflowers over free crude arginine in the production process of L-ornithine biotransformation.
4 Discussion
The preparation of L-ornithine using arginase has been extensively reported [7, 8, 26]. However, due to the limited stability of free enzymes, prolonged reaction times lead to gradual enzyme deactivation, affecting the efficiency of L-ornithine synthesis. Immobilized enzymes can effectively enhance the stability of arginase and facilitate the separation and recycling of enzymes and substrates/products after the reaction, thereby reducing the cost of enzymes. Traditional commercially available immobilized enzymes are typically prepared using organic resin [27,28,29,30]. The preparation process is cumbersome, and the immobilization process usually relies on covalent cross-linking, directly affecting the enzyme’s structure and its activity. Organic-inorganic hybrid nanoflowers offer a carrier-free immobilization method, requiring only simple materials such as phosphate buffer, metal ions, and crude enzymes. The preparation involves a straightforward three-step process of mixing, incubation, and solid-liquid separation to obtain immobilized enzymes. This immobilization method minimally impacts the enzyme’s structure and has a weaker effect on activity disruption, potentially leading to a significant increase in enzyme activity [31, 32]. Therefore, this method boasts advantages such as simplicity, reduced pollution, and low cost, making it a promising approach for widespread application.
However, enzyme purification generates a significant amount of saline wastewater, and purified enzymes require strict storage conditions. The use of pure enzymes for biotransformation in practical production is uneconomical and environmentally unfriendly. Therefore, it is often more economical and environmentally friendly to use whole-cell extracts instead of pure enzymes. In our attempts to prepare nanoflowers using whole-cell extracts, we observed that crude enzyme nanoflowers exhibited similar characteristics to pure enzyme nanoflowers. For instance, excessively high total protein concentrations reduced the nanoflower’s activity, and the formation of nanoflowers required a period of time. Additionally, an appropriate concentration of nickel ions could activate arginase nanoflower activity to a level similar to that of crude enzymes. This indicates that preparing crude enzymes as nanoflowers for bioreactions is entirely feasible. Furthermore, crude enzyme solutions contain a diverse range of protein types and ion species. Using traditional immobilization materials may result in reduced effective loading rates and increased carrier costs. This further highlights the advantages of the nanoflower method over traditional immobilized enzymes in terms of convenience and cost-effectiveness.
The organic-inorganic hybrid nanoflower immobilization method requires the involvement of metal ions, and for many metal-dependent enzymes, utilizing the metal ions they depend on results in the most effective formation of nanoflowers [14, 33, 34]. However, this rule does not apply universally, for example, magnesium ions and phosphate are not conducive to forming nanoflower structures. In cases where enzyme-based hybrid nanoflowers dissolve or undergo significant changes in properties in the reaction solution, the application of traditional nanoflower methods is restricted. In this study, we adopted two different metals during nanoflower preparation and enzyme activation. Calcium ions, which have no impact on activity and enable the stable existence of nanoflowers in arginine solutions, were used during nanoflower preparation. During catalytic reactions, nickel ions with strong activation effects were introduced, cleverly resolving the issues above. This strategy can provide valuable insights for enzymes that were originally limited by the inability to utilize metal ions for nanoflower preparation. Enzymes facing such limitations can potentially benefit from this approach, expanding the application scope of nanoflowers strategy in enzyme immobilization.
The preparation of organic-inorganic hybrid nanoflowers relies on coordination bonds formed between metal ions and organic components. If there are smaller molecular components in the reaction system with stronger coordination abilities, they may compete with the organic components in nanoflowers for metal ions, disrupting the original nanoflower structure and causing the nanoflowers to dissolve. In this study, we systematically investigated the impact of twenty natural protein amino acids on the solubility of nanoflowers formed with calcium, manganese, cobalt, nickel, and copper ions. Overall, the influence of amino acids on the solubility of these nanoflowers follows this trend: Ca > Mn > Zn > Co > Cu > Ni. Conclusively, the enzyme-inorganic hybrid nanoflowers prepared using calcium or manganese hold promise for applications in reaction systems containing amino acids due to their stability in various amino acid solutions. The results of this research provide significant insights into the development of enzyme-inorganic hybrid nanoflower immobilization strategies in amino acid-rich reaction systems, which can facilitate the broader application of this strategy in this specific reaction system. Furthermore, it offers research avenues for the development of enzyme-inorganic hybrid nanoflower immobilization strategies in other reaction systems containing compounds with strong coordination effects.
Data Availability
No datasets were generated or analysed during the current study.
References
M. Sivashanmugam, J. Jaidev, V. Umashankar, K.N. Sulochana, Biomed. Pharmacother. 86, 185 (2017). https://doi.org/10.1016/j.biopha.2016.12.024
R.F. Butterworth, A. Canbay, Dig. Dis. Basel Switz. 37, 63 (2019). https://doi.org/10.1159/000491429
L.B. Vong, Y. Ibayashi, Y. Lee, D.-N. Ngo, Y. Nishikawa, Y. Nagasaki, J. Control Release Off J. Control Release Soc. 310, 74 (2019). https://doi.org/10.1016/j.jconrel.2019.08.011
H.-A.A. Hussein, B.B. Mekki, M.E.A. El-Sadek, E E El Lateef Heliyon. 5, e02631 (2019). https://doi.org/10.1016/j.heliyon.2019.e02631
D. Harada, S. Nagamachi, K. Aso, K. Ikeda, Y. Takahashi, M. Furuse, Biochem. Biophys. Res. Commun. 512, 712 (2019). https://doi.org/10.1016/j.bbrc.2019.03.147
V.I. Shynkevych, S.V. Kolomiiets, I.P. Kaidashev, Heliyon. 7, e08353 (2021). https://doi.org/10.1016/j.heliyon.2021.e08353
Y. Zhan, J. Liu, P. Mao, H. Zhang, Q. Liu, Q. Jiao, World J. Microbiol. Biotechnol. 29, 2167 (2013). https://doi.org/10.1007/s11274-013-1382-5
T. Zhang, Y. Guo, H. Zhang, W. Mu, M. Miao, B. Jiang, Process. Biochem. 48, 663 (2013). https://doi.org/10.1016/j.procbio.2013.02.023
M.P. Naghshbandi, H. Moghimi, in Methods Enzymol, edited by C. V. KumarAcademic Press, (2020), pp. 431–451. https://doi.org/10.1016/bs.mie.2019.10.013
W. Tang, H. Li, W. Zhang, T. Ma, J. Zhuang, P. Wang, C. Chen, ACS Sustain. Chem. Eng. 10, 5384 (2022). https://doi.org/10.1021/acssuschemeng.1c07881
L.M.O. Arruda, M. Vitolo, Appl. Biochem. Biotechnol. 81, 23 (1999). https://doi.org/10.1385/ABAB:81:1:23
F.V.D. Velde, N.D. Lourenço, H.M. Pinheiro, M. Bakker, Adv. Synth. Catal. 344, 815 (2002). https://doi.org/10.1002/1615-4169(200209)344:8%3C815::AID-ADSC815%3E3.0.CO;2-H
Z. Lin, Y. Xiao, Y. Yin, W. Hu, W. Liu, H. Yang, ACS Appl. Mater. Interfaces. (2016). https://doi.org/10.1021/am502757e
Y. Chen, P. Liu, J. Wu, W. Yan, S. Xie, X. Sun, B.-C. Ye, X. Chu, J. Nanobiotechnol. 20, 347 (2022). https://doi.org/10.1186/s12951-022-01557-9
I. Ocsoy, E. Dogru, S. Usta, Enzyme Microb. Technol. 75–76 (2015). https://doi.org/10.1016/j.enzmictec.2015.04.010
X. Gao, S. Fang, X. Ma, T. Wang, C. Li, F. Lu, H.-M. Qin, Chem. Eng. J. 484, 149453 (2024). https://doi.org/10.1016/j.cej.2024.149453
Y. Zhang, C. Liu, S. Chen, C. Fan, Z. Jin, F. Yu, Mol. Catal. 559, 114056 (2024). https://doi.org/10.1016/j.mcat.2024.114056
N. Özdemir, C. Altinkaynak, M. Türk, F. Geçili, S. Tavlaşoğlu, Polym. Bull. 79, 9697 (2022). https://doi.org/10.1007/s00289-021-03973-7
Z.-F. Wu, Z. Wang, Y. Zhang, Y.-L. Ma, C.-Y. He, H. Li, L. Chen, Q.-S. Huo, L. Wang, Z.-Q. Li, Sci. Rep. 6, 22412 (2016). https://doi.org/10.1038/srep22412
Q. Liu, H. Shen, B. Li, J. Cai, Y. Peng, Z. Weng, H. Yu, G. Xie, W. Feng, Bioelectrochemistry. 146, 108152 (2022). https://doi.org/10.1016/j.bioelechem.2022.108152
B.S. Yilmaz, H. Bekci, A. Altiparmak, S. Uysal, İ. Şenkardeş, G. Zengin, Process. Biochem. 138, 14 (2024). https://doi.org/10.1016/j.procbio.2024.01.011
S. Dube, D. Rawtani, Adv. Colloid Interface Sci. 295, 102484 (2021). https://doi.org/10.1016/j.cis.2021.102484
J. Ge, J. Lei, R.N. Zare, Nat. Nanotechnol. 7, 428 (2012). https://doi.org/10.1038/nnano.2012.80F
F.P. Chinard, J. Biol. Chem. 199, 91 (1952). https://doi.org/10.1016/S0021-9258(18)44814-4
X. Zhang, J. Zhang, R. Zhang, Y. Guo, C. Wu, X. Mao, G. Guo, Y. Zhang, D. Li, Q. Zou, Int. J. Biochem. Cell. Biol. 45, 995 (2013). https://doi.org/10.1016/j.biocel.2013.02.008
K. Huang, S. Zhang, X. Guan, J. Liu, S. Li, H. Song, Appl. Microbiol. Biotechnol. 104, 6635 (2020). https://doi.org/10.1007/s00253-020-10721-w
H. K, M. K. A, N.T. F, S. K, S. C, S. T, T. H, T. T, and M. F, JP2009153448-A (16 July 2009).
Z. J and C. L, CN101914516-A; CN101914516-B (15 December 2010)
R.S.B.G.L. R, P.S.P. M, D.S.R. M, BR102017021316-A224 April (2019)
H. W, Z. X, and M. D, CN103205414-A; CN103205414-B (17 July 2013)
H. Jafari-Nodoushan, S. Mojtabavi, M.A. Faramarzi, N. Samadi, Adv. Colloid Interface Sci. 309, 102780 (2022). https://doi.org/10.1016/j.cis.2022.102780
F.P. da Costa, E.P. Cipolatti, A. Furigo, Junior, R. Oliveira, Henriques, Chem. Rec. 22, e202100293 (2022). https://doi.org/10.1002/tcr.202100293
R. Jian, Z. Tao, F. Qiu, Z. Yao, ACS Sustain. Chem. Eng. 5 (2017). https://doi.org/10.1021/acssuschemeng.7b00820
L.-B. Wang, Y.-C. Wang, R. He, A. Zhuang, X. Wang, J. Zeng, J.G. Hou, J. Am. Chem. Soc. 135, 1272 (2013). https://doi.org/10.1021/ja3120136
Author information
Authors and Affiliations
Contributions
Pengfu Liu: Conceptualization, Methodology, Validation, Writing and Supervision. Junying Fan: Investigation, Visualization and Writing-Original draft preparation. Xiaohe Chu: Supervision, Writing-Review & Editing, Funding acquisition.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Liu, P., Fan, J. & Chu, X. Immobilization of Arginase Using the Organic-Inorganic Hybrid Nanoflower Strategy for L-Ornithine Production. J Inorg Organomet Polym (2024). https://doi.org/10.1007/s10904-024-03268-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10904-024-03268-0