Abstract
The performance of the Lithium Ion Batteries (LiBs) is significantly influenced with the synergetic chemical properties of two different materials in a composite form. The specific capacity of both titanium dioxide arrays (TNAs) and Antimony trisulfide (Sb2S3) bottleneck the performance of LiB due to the low conductivity after the implantation as anode material. Herein, a novel multifunctional composite composed of highly dispersed Sb2S3 on freestanding tubular TNAs host via chemical bath deposition method (CBD) for use as anode material in lithium-ion batteries (LIBs). The loading quantity of Sb2S3 with reduced graphene oxide (rGO) was regulated to achieve adjustable outcomes. The composite anode consisting of TNAs/ Sb2S3/G in lithium-ion batteries (LIBs) has a specific capacity that is three times greater than conventional anodes. Furthermore, this composite anode maintains stable cyclic performance even after undergoing 300 cycles. The initial coulombic efficiency of the composite electrode is 100%, whereas the bare TNAs had a coulombic efficiency of 45%. The cycle performance analysis demonstrated that the TNAs/ Sb2S3/G composite has superior specific capacity and efficiency, even under high current density conditions of 500 µA/cm2. The rate performance is greatly improved, indicating the efficacy of this innovative composite anode material for high-performance LIBs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Lithium-ion batteries (LiBs) represent a groundbreaking frontier in energy storage technology, heralding a new era in the filed of energy-devices technologies [1]. Due to their high energy density, extended cycle life, and compact dimensions, these energy devices have attracted considerable interest as they have the potential to revolutionize other sectors, including wearable electronics and biomedical implants [2]. These batteries utilize the distinctive electrochemical characteristics of lithium ions at a small scale, providing exceptional prospects for enhancing portable electronics and facilitating inventive applications that require effective, lightweight, and durable power solutions [3]. The production of high energy density LiB are in high demand due to their multi-functional properties and uses, some due to the exceptional characteristics of lithium, and others due to the introduction of higher-performance anodes. These energy devices have become the most reliable and widely utilized storage medium, with applications ranging from portable to stationary. Lithium-ion batteries have unquestionably become the primary form of electrical energy storage for both portable electronics and vehicles. Their other features include easy manufacture, high energy density, a long life cycle, and stretchable designs [6,7,8,9,10]. . Such key properties are the primary reason for the attraction of lithium-based energy devices for use in portable devices and other biomedical equipment. LiBs are more forgiving than other types of micro-cells because they can withstand temperature variations, have lower cell voltage loss, and can maintain power delivery while in use. All of these properties are greatly appreciated, making LiBs a never-matchable power source [11,12,13,14].
The high capacity freestanding electrodes are in demand in different applications using LiBs as power source [4,5,6]. Typically, conductive carbon is used to increase electrode conductivity; however, additives are components that participate in undesirable reactions and are a specific cause of initial coulomb efficiency (IEC). The binder and carbon black-free electrodes are referred to as free-standing electrodes, which are in high demand in contemporary study [7, 8].
Free-standing composite electrodes are a popular research topic, although the majority of composites suffer from low conductivity. Titania nanotube arrays (TNAs) composites have been used as free-standing electrodes, however their conductivity is significantly impacted [9]. As is known, the electrode’s conductivity play a vital role in electron and lithium ion annihilation [10]. The conductivity of TNAs and its composites have been enhanced via different materials [11, 12]. The TNAs composite with high capacity materials and a blend of reduced graphene oxide (rGO) is a very efficient way for the success of free-standing anodes that is already being used [13].
Herein, We deliberately and effectively created a novel multifunctional composite by arranging highly dispersed Sb2S3 on a self-supporting tubular TNA host in a hierarchical manner. While rGO functions as both a conductive carbon material and an electrical connection between TNAs and Sb2S3. The combination of TNAs and Sb2S3 with a conductive rGO tubular matrix that has a significant pore volume and thin TNA walls ensures that this hierarchically nanoengineered composite has the potential to be an efficient anode material for lithium-ion batteries (LIBs).
2 Experimental Section
2.1 Synthesis
2.1.1 Synthesis of TNAs
TNAs electrode was fabricated by anodization of titanium foil as reported in our previous work [14,15,16,17,18]. First of all, 99.7% pure titanium foil (thickness ~ 0.125 mm, Sigma Aldrich) was degreased by sonication in acetone, ethanol, and water, respectively. Electrochemical anodization experiments were conducted at a constant potential difference between electrodes with a DC power supply (DH1722A-2 110 V/3A). The electrolyte solution was composed of 0.3 wt% NH4F and 2 vol% water in ethylene glycol (99.8%, anhydrous). All experiments were performed at room temperature. TNAs were grown at 50 V for 6 h and nanotubes layer was removed as reported [14]. TNAs were re-grown at same potential for 2 h and annealed at 450 °C in air for 2 h to transform from amorphous to anatase.
2.1.2 Synthesis of Antimony Trisulfide and Graphene Oxide Composite on TNAs
The Chemical Bath Deposition (CBD) method is used for the deposition of Sb2S3 and reduced graphene oxide (reduced GO) on TNAs [19, 20]. Firstly, antimony solution was prepared by adding 0.65 g of antimony chloride (SbCl3) to dissolved in 3 mL of acetone. Secondly, 1 mg/mL concentration dispersion of reduced GO was prepared by using a 400 W homogenizer in deionized water for 1 h. Thirdly, 1 mL of rGO dispersion was added to the antimony solution in acetone and stirred for 5 min. Now to prepare sulfur solution, 4 g sodium thiosulfate (Na2S2O3) is dissolved in 25 mL of deionized water and stirred for 5 min with magnetic stirrer and antimony solution is added into sulfur solution and stirred for further 10 min. Ice bath is used to maintain the temperature at approximately 4 °C. Then TNAs was placed face down into the solution for three hours, after three hours the sample is removed from the solution and clean with deionized water and placed in an oven at 60 °C.
TNAs/Sb2S3 sample was prepared for comparison by same method without mixing reduced GO solution. The synthesized samples of TNAs/Sb2S3 and TNAs/Sb2S3/G was annealed at 300 °C with a 5 °C/min rate in an argon atmosphere for 30 min to crystallize Sb2S3 layer.
2.2 Characterization
The morphology of TNAs and composites was characterized by using field emission scanning electron microscopy (FE-SEM LEO 1530) combined with an Energy Dispersive X-Ray (EDX) Analysis of system. The confirmation of TNAs, TNAs/Sb2S3, TNAs/Sb2S3/G phases was performed by using Cu Kα radiation (λ = 0.15 nm) of X-ray powder diffraction (Rigaku D/max).
2.3 Electrochemical Characterization
The electrodes of 1 cm2 and coupled with Li foils (0.25 mm thick and 99% purity) and assembled into R2025-type coin cells for electrochemical characterization. Prior to cell assembly, prepared electrodes were dried in a vacuum oven at 60 °C for 24 h to remove any residual solvent. A volume of 80 µL of 1 M LiPF6 in a 1:1 v/v mixture of ethylene carbonate (EC) and ethyl methyl carbonate (EMC) (Sigma Aldrich) was used as the electrolyte and battery-grade microporous membranes (Celgard® 2325, 25 μm thick) as separators.
Different TiO2 nanomaterials were studied for comparison. These electrodes were prepared by making slurry with additives to compare with free electrodes. TiO2 nanomaterials, carbon black and Polyvinylidene fluoride (PVDF) binder (80:10:10) were mixed in Vacuum mixer (TOB-ZKJB-150) overnight for homogeneous slurry. The mixed slurry was spread on copper Cu foil with doctor blade. The Cu foil was put in oven at 70 °C overnight to dry it completely to avoid moisture content. The coated foil was cut into electrode of 6 mm diameter which was cut by Disk Cutter (Xiamen TOB New Energy Co ltd China TOB CP60). The active materials were measured in analytical balance model TOB F A.1204B, China. All electrodes (free standing and slurry based) were heated overnight in a vacuum oven and send for battery assembly in glove box. All coin cells were assembled in an Ar-filled glove box with H2O and O2 content maintained < 0.5 ppm.
The Galvanostatic charge and discharge measurements were performed using a TOB CT4008, battery testing system. Battery charging and discharging were measured for a potential range of 0.005–3.0 V with respect to the Li/Li+. The rate performance was measured at various current densities. TNAs/Sb2S3 sample was prepared for comparison by same method without mixing reduced GO solution. The synthesized samples of TNAs/Sb2S3 and TNAs/Sb2S3/G was annealed at 300 °C with a 5 °C/min rate in an argon atmosphere for 30 min to crystallize Sb2S3 layer. The electrochemical impedance spectroscopy (EIS) measurement was recorded using electrochemical workstation (CHI660C, CH Instruments) at 5 mV s-1 between 0.01 Hz and 100 kHz.
3 Results and Discussion
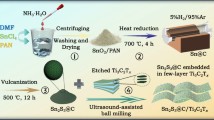
Figure 1 (a) depicts the scheme for TNA growth and coating. TNAs were created on Ti-foil, and then two distinct materials, rGO and Sb2S3, were placed to fill the tubes. Figure 1(b) shows that TNAs formed in two steps anodization had pore diameters of 60–100 nm and wall thicknesses of 20 nm. This technique produces high-quality TNAs that are regimented, vertically organized, and in the same phase. Figure 1(c) depicts coated TNAs, arranged and guided upright TNAs approaching a large region. Sb2S3 can embed well in such an individualized and tubular nanostructure. Figure 1(c) demonstrated that Sb2S3 is successfully implanted in TNAs.
EDS and EDX analysis identify the concentration and weight% of all other elements present in the composite. Figure 1(d) shows how much amount of carbon, oxygen, platinum, sulfur, and antimony present in the composite. Individually colored dots with high resolution appear to reveal the concentration of C, O, Pt, S and Sb in Fig. 1(c) and (d). Figure 1(c) shows the highest concentration of Sb of 29.07% in TNAs/Sb2S3 composite. The detailed mapping is also included in Supporting Information Figure S1.
The EDX of coated Sb2S3 and mixture with reduced GO were done which further confirm the successful coating. Table 1 shows the all the major and minor elements detected in TNAs/ Sb2S3, TNAs/Sb2S3/G which were indicated with weight% (wt%) and atomic % of elements.
The Energy Dispersive X-ray Spectroscopy (EDX) technique is used to identify and measure the components in samples, allowing for the calculation of the weight% of each component. The weight% of the TNAs/Sb2S3 composite provides evidence of the effective deposition of Sb2S3 onto TNAs. Figure 1(d) depicts the coexistence of carbon (C), sulfur (S), platinum (Pt), oxygen (O), sulfur (S), and titanium (Ti), indicating the development of TNAs/Sb2S3 composites. The inclusion of these elements in the composite is denoted by the vivid dots in the diagram. The identification of Ti and Sb elements is achieved by Energy Dispersive X-ray Spectroscopy (EDX), which corroborates the X-ray Diffraction (XRD) results and confirms the presence of Sb2S3 and reduced Graphene Oxide (GO). Figure 1(e) depicts the structural viewpoint of the TNAs/Sb2S3/G composite. The mixture was efficiently applied to TNAs and has the potential to function as the ideal freestanding anode for LIBs.
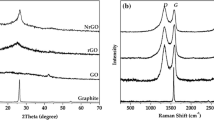
Figure 2(a) displays the XRD patterns of TNAs (anatase), TNAs/Sb2S3, and TNAs/Sb2S3/G composites. The application of heat at a temperature of 500 °C led to the creation of a polycrystalline tetragonal anatase structure, as shown from the XRD patterns. The X-ray diffraction (XRD) pattern of annealed titanium nanotube arrays (TNAs) in the presence of air can be categorized as anatase TNAs, according to the JCPDS 21-1272 classification. The X-ray diffraction (XRD) analysis of the titanium foil indicated that it is composed of many crystalline grains, as evidenced by the presence of distinct peaks at 38.05° and 40.04° (JCPDS no. 44-1294). The X-ray diffraction (XRD) pattern of the TNAs/Sb2S3 composite exhibited a prominent peak at an angle of 35.20°, corresponding to the presence of stibnite (crystalline Sb2S3 JCPDS No. 42-1393) [21]. The stibnite phase need meticulous annealing in a certified inert atmosphere.
The synthesized composites were analyzed using Raman spectroscopy, as shown in Fig. 2(b). The Raman spectra of pure anatase TiO2 nanoparticles exhibited characteristic peaks at 147 cm− 1 (Eg), 199 cm− 1 (B1g), 400 cm− 1 (A1g), and 639 cm− 1 (Eg), which were also observed in the Raman spectra of TNAs/Sb2S3/G hybrids. The Eg band of anatase TiO2 in TNAs/Sb2S3/G hybrids appeared to be blue-shifted (from 147 cm− 1 to 152 cm− 1), which could be attributed to the phonon confinement effect resulting from the different configuration of TiO2 nanoparticles in the heterostructures. The Raman modes associated with the crystalline structure of stibnite in the TNAs/Sb2S3/G samples were also detected, with two main Ag bands at approximately 285 cm− 1 and 313 cm− 1, corresponding to the Sb–S stretching modes related to the orthorhombic stibnite Sb2S3 phase. Additionally, for the rGO and TNAs/Sb2S3/G the graphene D and G bands were observed at 1325 cm− 1 and 1594 cm− 1, and at 1307 cm− 1 and 1574 cm− 1, respectively. The intensity ratios of D and G bands (ID/IG) were considerably higher (1.02 and 1.14) than the reference value of 0.84 obtained for graphene oxide, indicating enhanced reduction of the graphene oxide during the hydrothermal process and a high concentration of structure defects in the synthesized hybrids.
An optimal quantity of Sb2S3 and reduced GO mixture should be applied on the surface of TNAs to achieve the highest performance as an anode material. Electrochemical characteristics are influenced by surface parameters, such as smoothness, crystal structure, and roughness. The galvanostatic cycling test is employed to examine the performance of batteries. Figure 3(a), (b), and (c) were analyzed using a current density of 50 µA/cm2 and a potential range of 0.005–3 V. Figure 3(a) illustrates the process of discharging and charging during the initial cycle of TNAs, TNAs/Sb2S3, and TNAs/Sb2S3/G. The initial capacity values for TNAs, TNAs/Sb2S3, and TNAs/ Sb2S3/G in their first cycle are 429 µAh/cm2, 262 µAh/cm2, and 311 µAh/cm2, respectively. The data indicates that the specific capacity of the TNAs/Sb2S3/G composite increases with higher voltage, in comparison to TNAs or TNAs/ Sb2S3. The combined effect of TiO2 and Sb2S3 enhances the specific capacity, whereas the inclusion of reduced GO enhances electronic conductivity. Carbon black plays a crucial role in minimizing the mixing of GO due to the electrode being freestanding. Figure 3(b) illustrates the specific capacities of the last (300th) cycle, which are 39 µAh/cm2 for TNAs, 146 µAh/cm2 for TNAs/Sb2S3, and 178 µAh/cm2 for TNAs/Sb2S3/G. The bare TNAs exhibit a significant fading capacity of 390 µAh/cm2, but TNAs/Sb2S3 demonstrate a lower fading capacity of 116 µAh/cm2 and TNAs/Sb2S3/G exhibit a somewhat higher fading capacity of 133 µAh/cm2. Following 300 cycles, the capacity retention rates for TNAs, TNAs/Sb2S3, and TNAs/Sb2S3/G are 8.8%, 55.7%, and 57.2%, respectively. The rise in retention demonstrates that applying a suitable coating material not only enhances the capacity of TNAs, but also prolongs the lifespan of the battery.
Electrochemical Galvanostatic performance of TNAs, TNAs/Sb2S3, TNAs/Sb2S3/G at current density of 50 µA/cm2 at potential window of 0.005–3 V (a) First discharge and charge cycle and (b) Last discharge and charge cycle (c) Cyclic Performance and Efficiency (d) Rate Performance at the current densities of 50 µA/cm2, 200 µA/cm2, 500 µA/cm2, 50 µA/cm2
Figure 3(c) exhibits the cyclical performance and efficiency of TNAs, TNAs/Sb2S3, and TNAs/Sb2S3/G. The stability of TNAs decreases gradually from the first to the 300th cycle, resulting in a loss of 1.3 capacity per cycle. It is evident that the loss of capacity is more pronounced, particularly in the initial cycles, with a noticeable decrease in capacity up to the 100th cycle. Additionally, we note that the rate of capacity loss stabilizes notably after the initial cycles, as evidenced by the data representation in Fig. 3(c). The stabilization trend becomes more apparent, especially in the latter cycles, where the loss of capacity tends to plateau, indicating a more consistent performance over prolonged cycling. However, for TNAs/Sb2S3 composites, the cycle performance remains relatively stable from the first to the 300th cycle, with losses of 0.39 and 0.47 capacity per cycle for TNAs/Sb2S3/G, respectively. TNAs/Sb2S3 exhibit superior cycling stability compared to TNAs and TNAs/Sb2S3/G. However, due to their bigger capacity and reduced degradation, TNAs/Sb2S3/G demonstrate the maximum capacity in the final cycles. The inclusion of lithium particles in the TNAs/Sb2S3/G composite enhanced the process of adding and removing lithium, leading to a greater capacity compared to TNAs alone. The battery performance of anode materials is significantly affected by their initial coulombic efficiency (ICE). The usage of ICE is unfavorable because of the substantial quantity of lithium employed in the synthesis of SEI layer, as well as the occurrence of side reactions and the formation of stable side products. The internal conversion efficiencies (ICE) of TNAs, TNAs/Sb2S3, and TNAs/Sb2S3/G were calculated to be 45%, 97%, and 105%, respectively. The increase in ICE is attributed to the application of a protective artificial layer consisting of a mixture of Sb2S3 and reduced GO. This layer reduces the contact area of TNAs and also facilitates the creation of surface functional groups. The occurrence of high initial coulombic efficiency (ICE) in early charge-discharge cycles is common in electrodes that contain carbon, mostly because of the heightened reactivity between lithium and carbon materials within this voltage range. After a few cycles, the efficiency graph for all electrodes eventually hits 100% during galvanostatic discharge/charge [22].
Figure 3(d) shows rate performance of TNAs, TNAs/Sb2S3, TNAs/Sb2S3/G. The rate performance of TNAs, TNAs/Sb2S3 and TNAs/Sb2S3/G were measured at the current densities of 50 µA/cm2, 200 µA/cm2, 500 µA/cm2 and again at 50 µA/cm2.
The stability of TNAs, TNAs/ and TNAs/Sb2S3/G cycles eradicate progressively from lower to higher current densities and when return to lower density again the performance did not reduce. The lithium particle inclusion in mixture of Sb2S3 and TNAs with reduced GO (TNAs/Sb2S3/G) upgraded the lithiation/de-lithiation, which brings about higher limit than TNAs (Fig. 4).
The variables Rs, Rct, and W represent the bulk resistance of the electrode/electrolyte, the charge transfer resistance of the active materials/electrolyte, and the Warburg impedance (which represents the diffusion rate of Li+ in the host material under semi-finite conditions). The aforementioned values were derived through the process of semi-circle fitting using ZView software. The values of Rs, Rct, and W for TNAs/Sb2S3/G exhibited a drop from 1.477 to 1.174 Ω, 465.5 to 397 Ω, and 0.0065 to 0.0035 Ω, respectively. An increase in electronic conduction was expected by the decrease in resistance levels. Under identical experimental settings and with the same electrolyte, the values of Rs and Rct can be employed to assess the electric conductivity and the capacity of Li+ to diffuse through the interface layer between the solid and electrolyte, respectively. Consequently, the application of a Graphene coating on Sb2S3 in TNAs leads to an enhancement in electrical conductivity, as the resistance (Rs) reduces. Additionally, the diffusion of Li+ is improved, resulting in faster kinetics for electrochemical reactions. Consequently, the utilization of TNAs/Sb2S3/G as anode material has led to the attainment of increased specific capacity and remarkable rate capability in LIBs.
4 Conclusions
The functionality of Lithium-ion Batteries (LiBs) is intrinsically connected to the synergistic material composition of two distinct materials in a composite configuration. Antimony trisulfide was mixed with reduced graphene oxide to enhance the conductivity of the anode material. This mixture was then applied onto titania nanotube arrays using a cold bath approach. The utilization of the composite TNAs/Sb2S3/G as anode material led to a significant enhancement in performance. The cycle performance demonstrated that the TNAs/ Sb2S3/G composite exhibited superior specific capacity and efficiency, even under the demanding conditions of a high current density of 500 µA/cm2. This composite has a notable specific capacity of approximately 311 µAh/cm2, a coulombic efficiency of over 100%, and maintains consistent performance even under high current densities. These characteristics render it well-suited for practical applications in lithium-ion batteries. It is concluded that decorating titania nanotube arrays with antimony trisulfide and decreased graphene oxide increased the conductivity of the anode material. Because of the findings, these nano-arrays can be used in LiBs. The introduction of lithium particles in a mixture of Sb2S3 and TNAs with reduced GO (TNAs/Sb2S3/G) improved the lithiation/de-lithiation, resulting in a higher limit than TNAs. Scientifically, the current form of TNAs/Sb2S3/G-based LiBs reported here has the potential to play a dynamic role in the design and manufacture of lightweight and flexible LiBs.
Data Availability
Data will be made available upon request.
References
J.H. Pikul, H. Gang Zhang, J. Cho, P.V. Braun, W.P. King, High-power lithium ion microbatteries from interdigitated three-dimensional bicontinuous nanoporous electrodes. Nat. Commun. 4(1), 1732 (2013)
B. Hu, X. Wang, Advances in micro lithium-ion batteries for on-chip and wearable applications. J. Micromech. Microeng. 31(11), 114002 (2021)
Y. Wang, B. Liu, Q. Li, S. Cartmell, S. Ferrara, Z.D. Deng, J. Xiao, Lithium and lithium ion batteries for applications in microelectronic devices: a review. J. Power Sources. 286, 330–345 (2015)
S. Aslam, R.U.R. Sagar, Y. Liu, T. Anwar, L. Zhang, M. Zhang, N. Mahmood, Y. Qiu, Graphene decorated polymeric flexible materials for lightweight high areal energy lithium-ion batteries. Appl. Mater. Today. 17, 123–129 (2019)
L. Liu, M. Zhu, S. Huang, X. Lu, L. Zhang, Y. Li, S. Wang, L. Liu, Q. Weng, O.G. Schmidt, Artificial electrode interfaces enable stable operation of freestanding anodes for high-performance flexible lithium ion batteries. J. Mater. Chem. A 7(23), 14097–14107 (2019)
R.U.R. Sagar, N. Mahmood, F.J. Stadler, T. Anwar, S.T. Navale, K. Shehzad, B. Du, High Capacity Retention Anode Material for Lithium Ion Battery. Electrochim. Acta. 211, 156–163 (2016)
H. Chen, Y. Yang, D.T. Boyle, Y.K. Jeong, R. Xu, L.S. de Vasconcelos, Z. Huang, H. Wang, H. Wang, W. Huang, H. Li, J. Wang, H. Gu, R. Matsumoto, K. Motohashi, Y. Nakayama, K. Zhao, Y. Cui, Free-standing ultrathin lithium metal–graphene oxide host foils with controllable thickness for lithium batteries. Nat. Energy (2021)
F. Zhao, Y. Wang, X. Zhang, X. Liang, F. Zhang, L. Wang, Y. Li, Y. Feng, W. Feng, Few-layer methyl-terminated germanene–graphene nanocomposite with high capacity for stable lithium storage. Carbon. 161, 287–298 (2020)
R. Tjandra, G. Li, X. Wang, J. Yan, M. Li, A. Yu, Flexible high performance lithium ion battery electrode based on a free-standing TiO2 nanocrystals/carbon cloth composite. RSC Adv. 6(42), 35479–35485 (2016)
L. Li, D. Zhang, J. Deng, Y. Gou, J. Fang, H. Cui, Y. Zhao, M. Cao, Carbon-based materials for fast charging lithium-ion batteries. Carbon. 183, 721–734 (2021)
T. Song, U. Paik, TiO2 as an active or supplemental material for lithium batteries. J. Mater. Chem. A 4(1), 14–31 (2016)
J. Xia, X. Zhang, Y. Yang, X. Wang, J. Yao, Electrospinning fabrication of flexible, foldable, and twistable Sb2S3/TiO2/C nanofiber anode for lithium ion batteries. Chem. Eng. J. 413, 127400 (2021)
R.U.R. Sagar, M. Namvari, S.T. Navale, F.J. Stadler, Synthesis of scalable and tunable slightly oxidized graphene via chemical vapor deposition. J. Colloid Interface Sci. 490, 844–849 (2017)
L. Tauseef Anwar, L. Wang, X. Tongxiang, R.U.R. He, K. Sagar, Shehzad, Effect of aspect ratio of Titanium Dioxide Nanotube arrays on the performance of Lithium Ion Battery. Int. J. Electrochem. Sci. 10(7), 6537–6547 (2015)
T. Anwar, L. Wang, L. Jiaoyang, W. Chen, R.U.R. Sagar, L. Tongxiang, Lithium storage study on MoO3-grafted TiO2 nanotube arrays. Appl. Nanosci. (2016) 1–9
T. Anwar, L. Wang, R.U.R. Sagar, F. Nosheen, K. Shehzad, N. Hussain, L. Tongxiang, Molybdenum disulfide grafted titania nanotube arrays as high capacity retention anode material for lithium ion batteries. Appl. Nanosci. 7(1), 67–73 (2017)
T. Anwar, W. Li, R.U.R. Sagar, F. Nosheen, R. Singh, H.M. Jafri, K. Shehzad, L. Tongxiang, Cathodic Titania nanotube arrays as anode material for lithium-ion batteries. J. Mater. Sci. 52(8), 4323–4332 (2017)
T. Anwar, R.U.R. Sagar, S. Sheraz, F. Nosheen, S. Aslam, S.N. Shah, S.S. Ali, Y. Hui, T. Liang, Titania nanotube array decorated in polymer matrix as a free-standing anode material for lithium-ion batteries. Mater. Today Commun. 26, 101760 (2021)
P. Molaei, I. Kazeminezhad, One-step in situ synthesis of antimony sulfide/reduced graphene oxide composite as an absorber layer with enhanced photocurrent performances for solar cells. J. Nanopart. Res. 21(3), 54 (2019)
Y.-T. Song, L.-Y. Lin, Y.-S. Chen, H.-Q. Chen, Z.-D. Ni, C.-C. Tu, S.-S. Yang, Novel TiO2/Sb2S3 heterojunction with whole visible-light response for photoelectrochemical water splitting reactions. RSC Adv. 6(54), 49130–49137 (2016)
G.G. Bessegato, J.C. Cardoso, B.F. Silva, M.V.B. Zanoni, Enhanced photoabsorption properties of composites of Ti/TiO2 nanotubes decorated by Sb2S3 and improvement of degradation of hair dye. J. Photochem. Photobiol., a 276, 96–103 (2014)
Y. Gao, S. Chen, D. Cao, G. Wang, J. Yin, Electrochemical capacitance of Co3O4 nanowire arrays supported on nickel foam. J. Power Sources. 195(6), 1757–1760 (2010)
Acknowledgements
Authors worked in COMSATS batteries labs established by Higher Education Commission, under Research and Development Division, National Research Program for Universities (10640/Federal/ NRPU/R&D/HEC/ 2017).
Funding
This research has been carried out under grant No: 10640/Federal/NRPU/R&D/HEC/ 2017.
Author information
Authors and Affiliations
Contributions
Synthesis, methodology and write-up : Tauseef Anwar, Tareq Manzoor, Analysis: Naveed Hussain, Shazia Perveen and Syed Nasir Shah. Software and data, Sana Ullah Asif,Farhat Nosheen, Abdul Jabbar Khan, Habib Ullah Manzoor. Revision: Syed Nasir Shah, Tauseef Anwar.
Corresponding author
Ethics declarations
Competing Interests
There is no conflict of interest in this submission.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Anwar, T., Manzoor, T., Hussain, N. et al. Antimony Trisulfide with Graphene Oxide Coated Titania Nanotube Arrays as Anode Material for Lithium-ion Batteries. J Inorg Organomet Polym (2024). https://doi.org/10.1007/s10904-024-03135-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10904-024-03135-y