Abstract
Organic solar cell (OSC) devices suffer from low power conversion efficiency (PCE) due to deep trap states that absorb photogenerated charges, act as recombination centers, and reduce charge collection. To further improve the PCE, an optimized hole transport layer (HTL) must be engineered for efficient hole transport and reduced deep trap states. A hybrid HTL consisting of organic and inorganic materials was formed using a combination of CuO nanoparticles (NPs) having an average size of 50 nm and poly(3-hexylthiophene) (P3HT). The hybrid HTL was used in the fabrication and characterization of an inverted OSC device structure. In consequence, the incorporation of CuO-NPs into the P3HT resulted in an increase of 8.66% in photocurrent density. Additionally, the PCE increased from 3.77 to 4.22% while the open circuit voltage (Voc) was maintained. The higher hole mobility obtained for the optimum device using the charge extraction by increasing voltage (CELIV) measurement was 8.47 × 10− 4 cm2 V− 1 s− 1. This highlights the potential of designing novel organic solar cells with inorganic nanoparticles, offering enhanced performance and efficiency.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
In recent decades, renewable and sustainable energy has become one of the most prominent study areas. This field of research has grown in prominence as a result of the need to minimize greenhouse gas emissions and the impacts of climate change. Organic Solar cells (OSCs) made of carbon-based compounds have proven to be a feasible renewable energy source due to their cheap, flexibility, semitransparency, and ease of fabrication [1,2,3]. The organic materials used to make solar cells are non-toxic, making them an eco-friendly choice. Additionally, they can be produced quickly and cheaply, making them a great alternative to traditional silicon-based solar cells. Several methodologies have been used to improve OSCs’ performance, including the use of ternary active layers, the development of donor and acceptor materials, interfacial modification of carrier transport layers, and the standardization of manufacturing processes to improve carrier transport layers [4,5,6,7,8,9,10,11]. The bulk heterojunction (BHJ) concept allows for charge separation, which is essential for efficient charge transport in OSCs. The BHJ active layer also facilitates the formation of a nanoscale morphology that increases the surface area and improves the contacts between the electron transport layer (ETL) and hole transport layer (HTL) [12, 13]. The ETL and HTL are designed to further enhance this charge transfer by providing an efficient pathway for electrons and holes to move through the device. Additionally, OSCs incorporate organic, inorganic, and carbon-based layers as interfacial layers. These layers play a critical role in determining the performance and longevity of OSCs [14]. Poly-3-hexylthiophene (P3HT) is a widely-used material for organic electronics applications due to its impressive characteristics, such as its high charge carrier mobility, thermal and environmental stability, and solution processability [15, 16]. In this context, many metal oxide nanoparticles (NPs) such as CuO [17], NiO [18], and others have been added to P3HT to increase hole mobility. Among these inorganic NPs, CuO-NPs, have a 1.5 eV energy band gap, higher optical absorption, and provide more electrons into the structure [19, 20]. The numerous uses of CuO-NPs with controlled size, shape, and dopant have attracted many researchers. By providing electrons from CuO-NPs, the bandgap of P3HT can be narrowed, which can help to improve the efficiency of organic solar cells. Additionally, the addition of NPs can help to reduce the recombination rate between electron-hole pairs, which can lead to an increased charge-carrier lifetime.
However, with the incorporation of larger amounts of metal oxide nanoparticles into organic semiconductors, additional trap states may be formed in the band gap. They act as electron-hole recombination sites, leading to a decrease in the PCE of the OSCs [21, 22]. Understanding the density of trap states and how they can be prevented is essential to develop better-performing semiconductor devices. Siddiqui H, Parra M, et al. synthesized CuO-NPs using the wet chemical method and described their structural, and optical properties. The incorporated CuO-NPs into P3HT: PC70BM exhibit a higher efficiency of 3.82% due to efficient excitation, better light absorption, and photoexcited charge separation [17].
In this work, A multilayer OSC based on P3HT and CuO-NPs as a HTL was studied. Various ratios of CuO-NPs were added to P3HT to enhance hole extraction. The addition of 5 wt% CuO-NPs to P3HT resulted in an increased efficiency of 4.22% due to improved light absorption, and enhanced charge separation and extraction. Using the dark-CELIV method, the hole mobility of the optimum device was measured to be 8.47 × 10− 4 cm2 V-1 sec-1. The filling the transport layer with inorganic NPs, the risk of trap states increasing in the photoactive layer is further reduced. Moreover, this provides the advantage of improved electrical conductivity and stability in the device. A large excess of CuO-NPs in the P3HT layer beyond 5 weight % slowed the hole mobility due to trap-assisted recombination caused by deep trap states. These trap states can act as recombination centers, where charge carriers (electrons and holes) can recombine and be lost from the system before they can contribute to the current. This leads to a decrease in the hole mobility and, therefore, a decrease in the device performance.
2 Experimental
2.1 Materials
zinc acetate dihydrate Zn (CH3COO)2 2H2O, 2-methoxy ethanol CH3OCH2CH2OH (anhydrous, 99.8%), 2-Aminoethyl alcohol NH2CH2CH2OH, 1,2-dichlorobenzene (DCB, anhydrous 99%, C6H4Cl2), regioregular poly(3-hexylthiophene-2,5-diyl) (P3HT, (C10H14S)n), and copper oxide nanoparticles (CuO-NPs < 50 nm) were all purchased from Sigma-Aldrich. Patterned indium-tin-oxide (ITO, 25×20×0.7 mm, 9–15 Ω/sq) coated glass substrates were purchased from Lumtec, Taiwan. [6, 6]-phenyl C61-butyric acid methyl ester (PC61BM) C72H14O2 was purchased from Ossila. No additional purification was performed on any of the chemicals.
2.2 Zinc Oxide (ZnO) Synthesis
The layer of sol-gel ZnO was synthesized by dissolving 500 mg zinc acetate dihydrate in 4.875 ml 2-methoxy ethanol, adding 0.125 ml monoethanolamine as a stabilizer, and stirring the solution for 12 h in the air [23].
2.3 OSC Device Fabrication
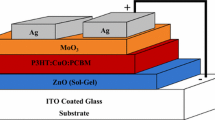
The inverted OSC devices are built on a transparent cathode of ITO-coated glass. ITO-coated substrates were firstly ultrasonically rinsed for 10 minutes in boiling deionized water, isopropyl alcohol, and acetone. The washed ITO-coated substrates were exposed to an O2 plasma cleaner after drying at 100°C for 12 minutes. After filtering the ZnO precursor through a 0.45 µm polytetrafluoroethylene filter, it was spun-coated for 40 seconds at 3000 rpm onto ITO-coated substrates before being annealed for 10 minutes at 200°C. An active layer of 1:1 wt% P3HT: PC61BM in 1 ml DCB was stirred for 24 h at 60 °C. In the next step, the blend solution was filtered through a 0.45 µm polytetrafluoroethylene filter. After that, the P3HT: PC61BM solution was spun-coated onto the ZnO layer for 50 seconds at 600 rpm, and it was then annealed for 10 minutes at 110°C. Additionally, various concentrations of CuO-NPs (0.0, 2.5, 5.0, and 7.5 wt%) were dispersed and sonicated in DCB and added to a P3HT at 15 mg/ml. The P3HT: CuO-NPs solutions are spun-coated on top of the P3HT: PC61BM layers at 600 rpm for 1 minute, followed by 5 minutes of annealing at 110. In the final step, the coated substrates were placed into the thermal evaporation system for the deposition of MoO3 and Ag with a thickness of 7 and 100 nm respectively. Inverted OSC devices are schematically depicted in Fig. 1.
3 Characterization
The ITO substrate consists of four prepared cells, each having a 0.06 cm2 active area. The microstructures of the CuO NPs were examined using a transmission electron microscope (TEM, JEOL/ JEM/1011, Japan). With the aid of a Shimadzu XRD-6000 X-ray diffractometer equipped with a CuK1 X-ray diffractometer (= 1.54060), the crystalline characteristics of the layers were investigated. Measurements of the UV-visible absorbance of the prepared layers were conducted using a JASCO (V-630) UV-Vis spectrophotometer. AFM (flex, contact mode, Nano Surf C300 (version 3.5.0.31) software) was used to investigate the surface shape of CuO-NPs doped P3HT layers. The photovoltaic characteristics were measured using a Keithley 2450 source meter unit under a 100 mW/cm2 AM 1.5 G illumination. The DG1022U waveform generator was used to apply a linearly increasing voltage, or a voltage pulse, to the device, and the UTD2202CE oscilloscope was used to measure the current flowing through the device. This allowed for the determination of the extracted charge carrier mobilities and densities. The measurements were conducted at a constant room temperature of 25 oC and 27% relative humidity.
4 Results and Discussion
Transmission electron microscope (TEM) imaging was used to investigate the shape, size, and distribution of the CuO-NPs (Fig. 2). TEM image shows that the CuO-NPs are uniform and spherical. The diameter of the spherical CuO-NPs is less than 50 nm. The XRD pattern of the CuO-NPs (Fig. 3a) indicates the presence of the pure monoclinic phase of CuO-NPs since all peaks are well indexed with the JCPDS card number 00-005-0661. Figure 3b shows the XRD patterns of pristine P3HT and CuO-NPs incorporated P3HT layer. According to the XRD data (Fig. 3b), adding CuO-NPs to the P3HT alters its crystallinity. The crystallinity of P3HT can be enhanced by the addition of CuO-NPs [24]. This increase in crystallinity may have enhanced the PCE and absorbance of the devices [25]. In other words, a higher level of crystallinity in the P3HT material can result in more efficient charge transport, allowing for higher device performance.
Figure 4 shows 3-D AFM images of the nanoscale surface roughness of the bare P3HT and CuO-NPs doped P3HT layers spun-coated onto ITO-coated substrates. The morphology of the pristine P3HT layer is smooth with a root mean square (rms) roughness of 3.4 nm. This indicates that the P3HT layer is relatively uniform and has a low level of surface roughness. On the other hand, the rms roughness of the P3HT layers containing 2.5, 5.0, and 7.5 wt% CuO-NPs are 5.15, 8.03, and 11.26 nm, respectively. The higher roughness of the P3HT layers containing CuO-NPs indicates that the CuO-NPs are adhering to the P3HT layers and the presence of the CuO-NPs is causing an increase in the surface roughness of the layer. This is important because it means that the layer is more likely to be able to enhance the conduction, as the CuO-NPs provide additional pathways for holes to move through.
Figure 5a shows the UV-visible absorption of virgin P3HT and CuO-NPs inserted P3HT layer. The absorption peaks obtained at 500 and 610 nm are attributed to the π-stacking of the P3HT structure. It is likely that the peak at 500 nm is associated with the π - π * transition. This is because the π-π* transition energy of a P3HT polymer is usually found around 500 nm [26, 27]. The peak at 610 nm is attributed to the n-π* transition, which occurs when electrons are excited from the non-bonding orbitals to the π* orbitals. A significant increase in the intensity of the vibronic absorption peaks was observed as CuO-NPs concentrations increased. Furthermore, with the introduction of CuO-NPs, the observed peak has been shifted by 510 nm due to the disturbance of the structure of P3HT caused by CuO-NPs’ ability to capture light. The CuO-NPs in the P3HT improved the absorption. Additionally, the CuO-NPs acted as a light-trapping agent, which further increased the absorption. To calculate the optical energy band gap for both pristine and CuO-NPs incorporated P3HT films, Tuac’s equation has been applied by plotting the relationship between (αhυ)1/2 and the photon energy (Fig. 5b) [28,29,30]. Table 1 lists the optical indirect band gap values derived from Fig. 5b. Upon filling with CuO-NPs, the estimated optical energy gap for pure P3HT was reduced from 1.93 eV to 1.81 eV.
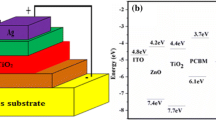
As a reference device, an inverted OSC device using P3HT: PCBM as an active layer sandwiched between ITO/ZnO and MoO3/Ag was manufactured and designated OSC0. A series of P3HT layers containing 0, 2.5, 5, and 7.5 wt % CuO-NPs were spun-coated interfacially between the active layer and MoO3 and were designated OSC1, OSC2, OSC3, and OSC4, respectively. Based on the formulas published in reports [31, 32], fill factor (FF), short circuit current density (Jsc), open circuit voltage (Voc), series resistance (RS), shunt resistance (RSH), and power conversion efficiency (PCE) were calculated and listed in Table 2. The J-V characteristic shown in Fig. 6a for the OSC0 device has been achieved with a PCE of 3.47%. However, the Jsc enhanced from 10.18 mA/cm2 to 10.78 mA/cm2 with the deposition of the bare P3HT layer. P3HT deposition results in improved performance largely due to improved band gap alignment and hole mobility, which speed up exciton dissociation at the active layer. This facilitates the efficient creation of free charge carriers, enabling faster charge transport and ultimately higher PCE. In the presence of CuO-NPs in the P3HT layer, the PCEs of OSC2, OSC3, and OSC4 were respectively 3.93, 4.22, and 3.21%. For instance, the PCE of OSC3 was seen to increase by 21.6% whenCuO-NPs were added to the P3HT layer. A maximum Jsc of 11.66 mA/cm2 and an FF of 60.27% was obtained with OSC3. Additionally, the series resistance (Rs) decreased from 238.44 Ω to 208.75 Ω. The significant improvement in OSC3 performance is primarily due to improved hole extraction from the active layer/P3HT to the anode electrode (Ag). This improved hole extraction means that the electrons can move more freely, resulting in faster electron transfer and improved OSC3 performance. Higher CuO-NPs inclusion into the P3HT layer reduced photovoltaic performance. This drop in OSC4 performance might be ascribed to the creation of deep-trap states, which impede charge carrier transit as illustrated in Fig. 6b.
Charge carriers’ density and mobility were examined in the dark using the CELIV approach to further explicate the reasons for device improvement and deterioration after inserting CuO-NPs incorporation. The hole mobility values were determined using the Juska formula [33]: The Juska formula uses the average drift velocity of a hole in a semiconductor to calculate the drift hole mobility.
where d is the thickness, A = V/\({\text{t}}_{\text{p}\text{u}\text{l}\text{s}\text{e}}\) represents the ramp voltage, \({\text{t}}_{\text{m}\text{a}\text{x}}\) is the time at the highest transient current, \(\varDelta \text{j}\) is the maximum extracted charge current, and j(0) is the displacement current ( j(0) = \(\epsilon {\epsilon }_{o} A/d\)). The A was set to be 2 × 104/3 Vs− 1, and the offset voltages ranged from 0 to 0.5 V. The charge carrier densities (n cm− 3) were calculated by integrating the CELIV transient at varied offset voltages, as illustrated in Fig. 7a. In conjunction with an increase in CuO-NPs in the P3HT layer, charge carriers were abundantly extracted from the devices. Based on Eq. (1), Fig. 7b illustrates the calculated hole mobilities at various voltage offsets. It is obvious that the incorporation of CuO-NPs improved the hole mobility value. For instance, at zero offset voltage, the highest hole mobility was 8.47 × 10− 4 cm2 V− 1 s− 1 for the OSC3 device. The CuO-NPs can form a continuous network that creates direct paths for the holes to travel through, thus improving hole mobility. This allows the holes to be efficiently extracted from the active layer and towards the Ag electrode, which increases the efficiency of the device. More additions of CuO-NPs increase the density of states near the conduction band, which results in the formation of deep trap levels. The deep traps capture the holes and prevent them from reaching the conduction band, thus inhibiting hole transport. The holes also recombine with electrons more quickly due to the presence of deep traps, which further reduces the hole transport.
5 Conclusion
CuO-NPs can absorb light energy and transfer it to the organic material, while poly(3-hexylthiophene) (P3HT) can effectively transport charge carriers to the electrodes and prevent charge recombination. Together, they create a highly efficient hybrid hole transport layer (HTL), allowing for the fabrication of an efficient multilayer OSC. The reference OSC device achieved a PCE of 3.47%. However, with CuO-NPs added to the P3HT layer at the optimal concentration, 4.22% PCE can be achieved due to the improved dissociation and collection of photoexcited charges. The CuO-NPs work as a hole transport material and act as a bridge between the active layer and the anode. The CuO-NPs also improve light absorption, leading to a greater number of photoexcited charges. Filling the transport layer with inorganic NPs further reduces the risk of increased trap states in the photoactive layer. The higher CuO-NP concentration increases the number of charge carriers that can become trapped in the shallow states, reducing the number of charge carriers available for transport and therefore reducing the overall transport rate.
Data Availability
The datasets used in this work are accessible from the corresponding author upon reasonable request.
References
Z. Hu, J. Wang, X. Ma, J. Gao, C. Xu, K. Yang, Z. Wang, J. Zhang, F. Zhang, Nano Energy. 78, 105376 (2020)
K. Fukuda, K. Yu, T. Someya, Adv. Energy Mater. 10, 2000765 (2020)
S. Peng, D. Wang, J. Lu, M. He, C. Xu, Y. Li, S. Zhu, J. Polym. Environ. 25, 1208 (2017)
X. Ma, J. Wang, Q. An, J. Gao, Z. Hu, C. Xu, X. Zhang, Z. Liu, F. Zhang, Nano Energy. 70, 104496 (2020)
W. Gao, Q. An, M. Hao, R. Sun, J. Yuan, F. Zhang, W. Ma, J. Min, C. Yang, Adv. Funct. Mater. 30, 1908336 (2020)
M. Zhang, F. Zhang, Q. An, Q. Sun, W. Wang, J. Zhang, W. Tang, Nano Energy. 22, 241 (2016)
P. Cheng, C. Yan, Y. Wu, J. Wang, M. Qin, Q. An, J. Cao, L. Huo, F. Zhang, L. Ding, Y. Sun, W. Ma, X. Zhan, Adv. Mater. 28, 8021 (2016)
Z. Hu, F. Zhang, Q. An, M. Zhang, X. Ma, J. Wang, J. Zhang, J. Wang, ACS Energy Lett. 3, 555 (2018)
S. Shah, M. Sayyad, K. Khan, J. Sun, Z. Guo, Nanomaterials. 11, 2151 (2021)
S.A.A. Shah, M.H. Sayyad, J. Sun, Z. Guo, J. Rare Earths. 40, 1651 (2022)
S.A.A. Shah, M.H. Sayyad, J. Sun, Z. Guo, Nanomaterials. 11, 48 (2020)
Y. Zhang, K. Liu, J. Huang, X. Xia, J. Cao, G. Zhao, P.W.K. Fong, Y. Zhu, F. Yan, Y. Yang, X. Lu, G. Li, Nat. Commun. 12, 4815 (2021)
S. Rafique, S.M. Abdullah, K. Sulaiman, M. Iwamoto, Renew. Sustain. Energy Rev. 84, 43 (2018)
L.C. Palilis, M. Vasilopoulou, A. Verykios, A. Soultati, E. Polydorou, P. Argitis, D. Davazoglou, Abd. R. bin Mohd Yusoff, and, M.K. Nazeeruddin, Adv Energy Mater 10, 2000910 (2020)
M.A. Deshmukh, S.-J. Park, B.S. Hedau, T.-J. Ha, Sol. Energy. 220, 953 (2021)
S.R. Bobbara, E. Salim, R. Barille, J.-M. Nunzi, J. Phys. Chem. C 122, 23506 (2018)
H. Siddiqui, M.R. Parra, P. Pandey, M.S. Qureshi, F.Z. Haque, J. Science: Adv. Mater. Devices. 5, 104 (2020)
E. Salim, J. Mater. Sci.: Mater. Electron. 32, 28830 (2021)
X. Miao, S. Wang, W. Sun, Y. Zhu, C. Du, R. Ma, C. Wang, Scr. Mater. 165, 134 (2019)
A. El-Trass, H. ElShamy, I. El-Mehasseb, M. El-Kemary, Appl. Surf. Sci. 258, 2997 (2012)
N.K. Elumalai, C. Vijila, R. Jose, A. Uddin, S. Ramakrishna, Mater. Renew. Sustain. Energy. 4, 11 (2015)
R. Medhi, M.D. Marquez, T.R. Lee, ACS Appl. Nano Mater. 3, 6156 (2020)
A. Magdy, A. El-Shaer, A.H. EL-Farrash, E. Salim, Sci. Rep. 12, 21489 (2022)
M. Ikram, M. Imran, J.M. Nunzi, Islah-u-din, and, S. Ali, Synth Met 210, 268 (2015)
M. Ikram, M. Imran, J.M. Nunzi, S. Ali, J. Mater. Sci.: Mater. Electron. 26, 6478 (2015)
H. Siddiqui, M.R. Parra, M.S. Qureshi, M.M. Malik, F.Z. Haque, J. Mater. Sci. 53, 8826 (2018)
E. Salim, S.R. Bobbara, A. Oraby, J.M. Nunzi, Synth. Met. 252, 21 (2019)
E. Salim, A.E. Tarabiah, J. Inorg. Organomet. Polym. Mater. 33, 1638 (2023)
E. Salim, W. Hany, A.G. Elshahawy, A.H. Oraby, Sci. Rep. 12, (2022)
A.M. Alshehari, E. Salim, A.H. Oraby, J. Mater. Res. Technol. 15, 5615 (2021)
A. Moliton, J.-M. Nunzi, Polym. Int. 55, 583 (2006)
M. Imran, M. Ikram, S. Dilpazir, M. Nafees, S. Ali, J. Geng, Appl. Nanosci. 7, 747 (2017)
G. Juška, K. Arlauskas, K. Genevičius, Lith. J. Phys. 56, 182 (2016)
Acknowledgements
The authors would like to thank Mansoura University for supporting their research with grant number MU-SCI-20-14. Open access funding is provided by Science, Technology & Innovation Funding Authority (STDF) in cooperation with the Egyptian Knowledge Bank (EKB).
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
M. Agha carried out the tests, drew the curves, and wrote the methodology. M. El-Kemary supervised the project and reviewed the manuscript. A. H. Oraby supervised the project and edited the text. E. Salim planned and carried out the tests, as well as wrote and reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Conflict of interest
The authors have reported no competing financial interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Agha, M., El-Kemary, M., Oraby, A.H. et al. Efficient Multilayers Organic Solar Cells with Hybrid Interfacial Layer-based P3HT and CuO Nanoparticles. J Inorg Organomet Polym 34, 557–564 (2024). https://doi.org/10.1007/s10904-023-02829-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10904-023-02829-z