Abstract
A novel core/shell nanoferrite material has been developed for use in various potential applications. Rare-earth doped calcium-ferrite with the compositional formula \(\text{Ca}{\text{Fe}}_{1.925}{\text{Sm}}_{0.05}{\text{Gd}}_{0.025}{\text O}_4\) was prepared as a core using the citrate auto-combustion method before being coated with PVA as a polymeric shell. High crystalline and single-phase orthorhombic nanoparticles are confirmed by XR-D (X-ray diffraction). An estimate has been made for the mean size of the crystal structures by analyzing the broadening of XR-D lines within a range of 20 nanometers. HR-TEM (high-resolution tunnelling electron microscope) micrographs also showed that the particles had an orthorhombic shape with well-defined boundaries. EDX (energy-dispersive X-ray) and FT-IR were used to investigate the elemental constitution and the molecular structure of samples. The dielectric properties were discussed in the basics of interfacial polarization and Koop`s model. The anti-ferromagnetic nature of the samples was identified by a VSM (vibrating sample magnetometer). The proposed composition was found to improve the electromagnetic absorption performance, which was confirmed with the measurements of optical parameters from the absorbance spectrum recorded by the UV–VIS–NIR spectroscope. The optical band-gap and Urbach energies of the synthesized samples have been investigated, in addition to the refractive index and the extinction coefficient. The Wemple-DiDomenico oscillator model was used to examine the dispersion energies.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
1 Introduction
Spinel nanoparticles (SNPs) with innovative designs have gained a lot of attention due to their extraordinary nanostructure in addition to their exceptional electronic and optical properties. Nanocrystalline ferrite has a diverse array of applications, including the delivery of anti-cancer medications that are controlled by magnetic fields [1] and as a contrast medium utilized in magnetic resonance-imaging (MRI) for detecting cancer and other diseases [2]. The study of ferrite nanostructures has increased significantly because of their potential application in magnetic storage with high-data density and hyperthermia-treatment [3, 4].
Current technological devices such as radar systems, mobile phones, solar energy harvesting, and wireless sensor equipment require electromagnetic absorbing material [5].
Such electromagnetic absorbent material is classified based on how it works, and its crucial features include a broad absorption bandwidth and light-weight. In order to meet these requirements, it is possible to use a combination of two or more nanomaterials to adjust the absorber’s parameters [6]. The unique electrical and optical properties of SNPs make them suitable for many domestic applications, such as energy research, catalysis, spintronics, and sensors [7, 8].
Core/shell NFs are competent candidates for numerous bio-medical applications including identifying and diagnosing early signs of cancerous growths and as a drug delivery agent. Additionally, they are currently used for removing heavy metals from soil and wastewater [9,10,11]. According to research, doped ferrite is considered to be an affordable and easily controlled material that has the potential to be utilized in a wide spectrum of applications, for instance, communications, electronic devices, solar-cells, radio-frequency devices and microwave devices [12, 13]. The development of doped ferrites by coating them with organic shell has the potential to create multi-functional devices for nanoscale opto–electro–mechanical systems, catalysts, logic operations, data-storage and particularly electronic components that can alternate between different functions, such as transistors, resistors, capacitors, and inductors allowing for multiple-functions to be achieved within a single system [14, 15]. Recently, ferrite-based nanomaterials coated with polymeric shells have been utilized to create electromagnetic absorbers because they offer a simple, low-cost, and customizable method for tailoring the emission spectrum, as well as being light-weight [16, 17].
When CaFe2O4 is formed at high calcination temperature, it takes on an orthorhombic unit cell structure. In this structure, large cations occupy A site, which is a twelve-fold site located at the corners of the orthorhombic unit-cell, while small cations sit in the B site, which is at the center of the six octahedral coordination of oxygen atoms [18]. Cubic symmetry distortion is common, including cation displacement in the \({\mathrm{BO}}_6\) octahedra and octahedral tilting. These distortions are influenced by the structural and electronic factors of the A and B sites elements [19]. The electrical and optical properties are influenced by the distribution of cations involving the charges, spin, and orbital interactions at the pair of metal location [20].
Ateia et al. [18] studied the characterization and physical properties of CaFe2O4 before and after doping with varying amounts of rare earth (RE) ions. As previously demonstrated in their study, the incorporation of Sm3+ and Gd3+ ions into spinel ferrite would result in structural deformation. Larger ionic radii of RE ions compared to Fe ions imply greater strain, which changed the magnetic and electrical properties. Additionally, the interaction of the unpaired electrons in the 4f subshell of RE ions with the 3d subshell of Fe ions s turns out the physical properties of the studied samples.
Furthermore, the optical bandgap was determined by Ateia et al. [21] for the uncoated samples. The band gaps for the allowed-indirect transition decreased from 1.6 to 0.9 eV for the parent and doped samples, respectively. Due to the relatively small energy gap, the authors recommended that the doped samples be used as good candidates for water treatment by the mere application of visible light.
In this study, rare-earth doped calcium ferrite \(\left(\mathrm{Ca}{\mathrm{Fe}}_2{\mathrm O}_4\right)\) is utilized in the core/shell structure owing to its physical properties, such as its high thermal and chemical stability [22].
This leads to adjustments in the nanostructure, electrical, and optical properties of the material [23]. The main objective of the present work is to prepare a core–shell nanostructure with enhanced physical properties. Accordingly, we proposed a synthesis plan, which involves two issues. The first one is synthesized \(\text{C}\text{a}{\text{F}\text{e}}_{1.925}{\text{S}\text{m}}_{0.05}{\text{G}\text{d}}_{0.025}{\text{O}}_{4}\) NPs by a citrate auto combustion procedure. The coating of long-chain PVA molecules on the prepared NPs is the second issue. XRD, HRTEM, and FT.IR are the main techniques used in the present study.
2 Experimental Techniques
2.1 Preparation Methods
2.1.1 Synthesized of \(\text{Ca}{\text{Fe}}_{1.925}{\text{Sm}}_{0.05}{\text{Gd}}_{0.025}{\text O}_4\)
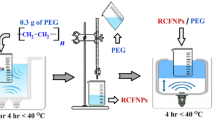
A novel nano ferrite with the formula \(\text{Ca}{\text{Fe}}_{1.925}{\text{Sm}}_{0.05}{\text{Gd}}_{0.025}{\text O}_4\) (CFRO) was synthesized by the citrate-auto-combustion technique to obtain NPs with a reduced size of calcium-ferrites as mentioned in the previous work [24]. The steps of the preparation technique are presented in Fig. 1. The obtained powder was subjected to calcination in a muffle-furnace at a high-temperature of 900 °C/4 h.
2.1.2 Preparation of Core–shell \(\text{Ca}{\text{Fe}}_{1.925}{\text{Sm}}_{0.05}{\text{Gd}}_{0.025}{\text O}_4@\text{PVA}\)
High-quality polyvinyl alcohol (PVA) with a molecular mass of 30 kg/mol was brought in by Indian LobaChemie Pvt. Ltd. This synthesized polymer was selected to coat CFRO in a core-shell structure, where CFRO NPs function as an inner core that is covered with a shell of polymer. The ultra-sonication technique is employed to achieve CFRO@PVA core-shell design.
In the present investigation, a solution containing 700 mg of CFRO was prepared by adding 25 ml of purified H2O to a beaker. In a separate beaker, 300 mg of the selected polymer was dissolved in 25 ml of purified H2O. Both beakers underwent sonication for approximately four hours. Subsequently, the solution of polymer was introduced into the CFRO solution, and the resulting mixture was subjected to sonic vibrations for a duration of four hours. The mixture was subjected to drying at a temperature of 60 °C for a duration of 20–24 h, resulting in the formation of a core–shell of CFRO/polymer [11].
2.2 Characterization Techniques
The X-ray diffraction spectroscope (XRD) from US Diano-Corporation, using a Cu sputtering target (λ = 1.54 Angstrom), is employed to evaluate the structure and crystallite size. The Scherer method is utilized for determining average NPs sizes. Additionally, an electron microscope that utilizes tunnelling electrons to achieve high resolution (HR_TEM: model JEIM-100 S) is utilized for examining surface morphology or microstructure. Morphological analyses are identified using a scanning-electron-microscope (SEM) with energy-dispersive X-ray analysis (EDX) and accelerating potential difference of 35 kilo volt. The molecular-characteristics were verified through Fourier-transformed-infrared (FT-IR) spectroscopy using an (IR) Affinity-1 S. Electronic characteristics are examined by LC-R measuring HIOKI/3511-50 in the frequency range of 100 kHz to 5 megahertz. The magnetic nature was determined by analyzing the hysteresis curves using the L-DJ model 9600-1 vibrating-sample-magnetometry (VSM). Optical characteristics are studied by applying a diffuse reflectance spectrometer in the ultraviolet, visible, and near-infrared regions (DR-S; design Shemadzu UV-V-NIR 3100PC).
3 Results and Discussion
3.1 Structure and Morphology
3.1.1 XR-D Analysis
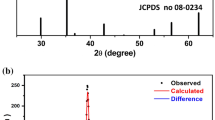
CFRO is synthesized effectively without the formation of any secondary phases. The chart of the prepared samples illustrates a range of 2θ (20° to 80° Fig. 2). The broadness of the peaks indicates the formation of NPs and is indexed to the orthorhombic unit cell structure with space group symmetry number 62 (Pnma). Importantly, the observed spectrum corresponds very closely to the standard spectrum of \(\text{Ca}{\text{Fe}}_2{\text O}_4\left(\text{ICDD 00-008-0100}\right)\) [25].
XR-D patterns of PVA display only one distinct peak at 19.25° indicating the semi-crystalline nature. This peak is also observed in the XR-D pattern of CFRO/PVA, implying the existence of PVA. The remaining peaks of the XR-D patterns for CFRO/PVA correspond to the orthorhombic unit cell structure of CFRO.
Using the Scherer approach, as described in earlier research [26], the mean crystallite sizes (D) of CFRO and CFRO/PVA are estimated to be 18 and 22 nm, respectively. The values obtained agree well with Ateia et al. [27], while the mean crystallite size for Co0.5Ni0.5Fe2O4/polyaniline nanocomposites was estimated by Gurusiddesh et al. to be twice these values [28].
The three independent lattice constants a, b and c are calculated based on the orthorhombic phase equation and presented in Table 1. The strain or compressive stress induced by the coating layer of polymer resulted in a slight decrease in lattice constant values [29].
3.1.2 HR-TEM Analysis
Figure 3 displays the HR-TEM micrographs of the CFRO and CFRO/PVA, which demonstrate that the particles are uniformly dispersed with well-defined boundaries and varied shapes. The particle size histograms obtained from measuring different particle sizes using Image-j software are presented in the figure [30]. The histograms show the particle size distribution within the range of 38:45 nanometers. The orthorhombic grain of CRFO exhibits low agglomeration with a homogeneous distribution, indicating good physical mixing.
It should be noted that the HR-TEM micrograph depends on the crystallography view plane, leading to the variety in the particle’s shapes. The characteristics of orthorhombic unit cell structure may be recognized by examining h, k, and l planes with lower Miller indices including 0 and 1, while, in some instances, equilibrium unit cell structure formation may involve surfaces with higher Miller indices[31].
CFRO/PVA HR-TEM micrographs clearly illustrate the shell thickness ranges from 1.5 to 4 nm. Additionally, it can be observed that the NPs exhibit uniform distributions with comparatively reduced clustering and aggregation after coating by polymeric shell, which prevents the agglomeration of NPs.
3.1.3 EDX Analysis
CFRO and CFRO/PVA particles are investigated using EDX analysis to determine their composition and stoichiometry. As shown from Fig. 4, strong signals are detected in the two samples for oxygen, calcium, iron, samarium, and gadolinium, while a carbon signal is detected only for CFRO/PVA, indicating the presence of PVA in the sample.
The measured and expected values of weight and atomic percentage are shown in the inset of the figure. These results provide evidence that the samples are successfully synthesized without any impurities.
3.1.4 FT-IR Analysis
FT-IR analyses confirmed the presence of the core CFRO, and the polymeric shell (PVP). Figure 5 displays the FT-IR spectra of the synthesized CFRO/PVP core/shell NPs. The FT-IR spectrum can be divided into two main regions CFRO-region: (core-region) and the polymer-region (PVA-region).
Within the CFRO-region, the bending-mode of Fe–O–Fe appears at the bands in the range of 478 to 524 cm−1. The deformations of FeO6 octahedral in orthorhombic unit cell can be represented at 571 cm−1. The asymmetric-stretch mode of Fe–O–Fe is appears at 648 cm−1, while the symmetric-stretch mode of Fe–O–Fe is appears at 714 cm−1 [18].
For the prepared sample of CFRO/PVA, within the PVA-region, the stretch-modes of O–H and C–H are detected at 3411 and 2916 cm−1, respectively. The bending-mode of C–H appears in the range 1427–1492 cm−1. Additionally, the stretching-mode C–C is represented at 853 [11]. The distinctive bands for PVA in CFRO/PVA occur at 1794 cm−1 which is attributed to the stretching-mode of C=O, in addition to the appearance of the stretching-mode C–O at 1084 and 1640 cm−1 [32].
The alcoholic O–H stretching band at 3260 cm−1 was observed in CoFe2O4 NPs coated with PVA. Sagayaraj et al. [33] discovered the extra bands at 2982 cm−1 relating to C–H stretching vibrations and at 1385 cm−1 corresponding to C–C stretching vibrations. The change that was detected is due to the preparation conditions and the type of core materials.
3.2 Electrical Properties
Figure 6 displays the variation of the real part of the dielectric constant ε′ with the absolute temperature as a function of the applied frequency for CFRO/PVA. From the figure, the ε′ is nearly constant with increasing temperatures up to about 600 K, this constancy of ε′ is due to the localization of dipoles at low temperatures [34], which is the normal behavior of magnetic semiconductors [35, 36]. The sudden increase of ε′ can be attributed to polarization at the interface or space charge [37], that is generated by the sequenced thermal release of multiple dipoles. This leads to the accumulation of charges at the interfaces in multiphase dielectrics, followed by their alignment in the electric field direction [38].
The decrease in ε′ following the peak is due to an increase in lattice vibration, which causes scattering of charge carriers as well as dipole disordering. According to Maxwell Wagner [39], the increase in ε′ after reaching the lowest value is due to the participation of additional types of polarization. This type of polarization is crucial in improving sample conductivity in the region separating the grains and increasing the dielectric constant due to increasing polarizability.
The difference in the values of ε′ between CFRO (inset figure) and CFRO/PVA is partially attributed to the difference in ionization energies between the magnetic core and the polymeric shell [40]. Accordingly, the smaller ionization energy of the elements may have a substantial impact on the polarization procedure. The figure shows a reduction of ε′ with increasing the frequency, which is expectable in the ferrites NPs. This behavior is also observed by earlier research [41]. The obtained behavior can be attributed to the relaxation response of the sample to applied electric field.
At lower frequencies, electron movement between ion-pairs of \(\text{Fe}^{3+}\to\text{Fe}^{2+}\) occurs in reaction to the applied electric field, which causes the alignment of the electric dipole in the electric field direction, leading to an increase ε′. In addition, there are two more factors that cause the high values of ε′ at lower frequencies. The first is the effective combination of space-charge, dipolar, ionic, and electronic polarization [42] and the second is that, Fe2+ ions are easily polarized compared to Fe3+ ions. At a specific high frequency, the hopping frequency of electrons between ions is unable to keep up with the variations in the applied electric fields because the rate of dipole rotations is not sufficient to match the change in the frequency of the applied electric field [43]. Therefore, the cation’s redistribution process gets stopped at all locations in nanoferrites and hence the interfacial polarization will be decreased. Finally, ε′ is becoming unaffected by higher frequency [44].
It is clear from the figure that, at low temperature, the values of the dielectric constant of CFRO/PVA are higher than those of CFRO before coating. The presence of the polymer inside the lattice can enhance the motion of electrons between Fe2+ and Fe3+ where the conduction mechanism and the polarization mechanism are the same and depend on the charge carriers and thereby decreases the dielectric constant [45, 46].
3.3 Magnetic Properties
The magnetization characteristics can be examined through the magnetization hysteresis curves (MH) for the samples, including the magnetic parameters. VSM records the hysteresis loops as shown in Fig. 7a, b. All samples display anti-ferromagnetic properties, as indicated by their distinctive non-saturating MH curves. This behavior may be explained by the confined area enclosed within the curve and the low magnetization value.
The magnetic nature of the samples is a result of indirect super-exchange magnetic interactions, between Fe3+ and Sm3+ through O2−. The saturation magnetization does not occur in the hysteresis loops due to the nature of their magnetic ordering, and the magnetic moments of anti-ferromagnetic samples align with neighboring moments [47]. When an external magnetic field is applied to an antiferromagnetic material, it can disturb the delicate balance between the atomic magnetic moments, causing a slight canting or tilting of the moments away from perfect antiparallel alignment. However, this tilting effect is limited and does not result in the alignment of magnetic moments along the field direction, as observed in ferromagnetic materials. As a result, antiferromagnetic materials do not exhibit a well-defined saturation magnetization. The magnetic hysteresis curves of antiferromagnetic materials typically show a much smaller range of magnetization compared to ferromagnetic materials [48].
Table 2 shows the magnetic parameters estimated from MH curves such as the saturation magnetization (\({M}_{s}\)), the retentivity \(\left({M}_{r}\right)\) and the coercivity \({H}_{c}\). The observed decrease in magnetization values after coating by polymeric shell, is attributed to the presence of a magnetic dead-layer of polymer on the surface.
Multi-domain states need less magnetic field to switch compared with single domain states. As shown in the figure, there is a slight decrease in \({M}_{s}\) for CRFO/PVA NPs compared with the uncoated ones. The same results were obtained by Omelyanchik et al. [49], who concluded that the core/shell systems showed an unchanged value of saturation magnetization compared to the core ferrite NPs.
The separation of each particle from its neighbors with the PVA shell reduces the magneto-static coupling between the particles, so the crystals will grow, and create multi domain states. These are the crucial issues behind the observed reduction in the magnetic parameters.
The \({M}_{s}\) is determined accurately according to the Stoner/Wohlfarth (S/W) model using the following equation:
where, M is the magnetization of the sample, H is applied magnetic field and β is a magnetic constant. The curve of magnetization versus \(1/{H}^{2}\) for CFRO, and CFRO/PVA can be extrapolated to approach zero, and hence \({M}_{s}\) can be estimated from the intercept value, as illustrated in Fig. 7c, d [50]. The obtained data agrees well with that estimated from the hysteresis loop measurements.
Switching field distribution (SF-D) refers to the spatial distribution or pattern of magnetic fields during the switching process in magnetic materials or devices. SF-D is important in various applications, such as magnetic storage devices (e.g., hard disc drives) and magnetic random-access memory (MRAM). Understanding and controlling the SF-D can help optimize the performance and reliability of these devices.
SF-D is calculated using the following equation [51].
Here, \({H}_{C}\) represents the coercive field, and \(\varDelta H\) is the full-width at half-maximum of the \(dM/dH\) plot, as depicted in Fig. 8. The dM/dH curve exhibits a doublet peak pattern, indicating the competition between strong dipolar-interactions and exchange-coupling [52].
The lower values of SF-D and greater values of coercive field of the samples (as indicated in Table 2) are ideal for achievinghigh recording performance. From Table 2, SF-D values clearly demonstrate that coating with a polymeric shell offers the benefit of decreasing the SF-D values, which are associated with optimal recording characteristics.
3.4 Optical Properties
The optical properties of CFRO and CFRO/PVA nanocomposites are examined using Ultraviolet Visible near-IR spectrophotometer. This spectroscopic technique involves recording the absorbance spectrum of light at various wavelengths in the range from 200 to 1100 nm at room temperature as shown in Fig. 9a, b. The absorbance values exhibit a substantial increase, reaching 10%, for light wavelengths below 250 nm in the ultraviolet (UV) domain. Beyond this point, the values of absorbance continue to rise gradually. This increase can be attributed to the electronic transitions of Fe (3d) and Ligand-Metal-Charge-transitions of the orthorhombic.
The determination of the optical-band-gap values (\({E}_{g}\)) of the studied samples is accomplished by calculating the fundamental-absorption-edge (FAE). FAE refers to the energy at which electrons transition from the valence-band (V.B.) to the conduction-band (C.B.). The Tauc Law is employed to evaluate Eg, utilizing the relationship between the absorption coefficient (α) and the incident photon energy E (E = h), as defined by Davis and Mott [53]
where \(h\) is the Planck constant, \(\upsilon\) is the vibration frequency of electromagnetic waves, and B is the relationship constant, which depends on the electronic transition probability. The value of the exponent (n) signifies the nature of transition, where n equals 1/2 and 2 for direct and indirect bandgaps, respectively.
The \({E}_{g}\) can be determined via Eq. 3 (inset in Fig. 9a, b). The calculated values of \({E}_{g}\) for CFRO, and CFRO/PVA are found to be 2.084, and 1.933 eV, respectively, which are larger than the value obtained by E. Ateia et al. for PEG coating spinel ferrites [11], and smaller than the value obtained by Zein K. Heiba et al. for PMMA/ CdMnFe2O4 [54]. As PVA coated the magnetic materials, the Eg decreased similar effect was observed in silica coated magnetic nanoparticles [55]. This, suggests that coating with a polymeric shell reduces the direct band gap energy value.
This reduction in the value of \({E}_{g}\) can be attributed to the formation of chemical bonding between PVA chains and CFRO ferrite NPs which is responsible for the generation of localized states between the HOMO and LUMO energy bands, making the lower energy transitions possible. This relies on the defects inside the material.
Urbach energy (\({U}_{E}\)) is mainly used to describe a characteristic of a nanoferrite (NF) absorption spectrum, which is also known as the Urbach tail or Urbach edge [56]. In some NFs, especially those with defects or impurities, additional absorption results from localized electronic states or structural disorder within the material at energy bands slightly lower than the band gap edge. The Urbach energy is related to the energy width of these tail states. In the core-shell structure, the CFRO and PVA have distinct properties.
The influence of the core-shell structure on \({U}_{E}\) in a particular system depends on the specific characteristics of the core-shell system, such as the size of CFRO and PVA, the interface quality, and the crystal lattice. The electronic properties, such as the bandgap, can be modified compared to the individual CFRO and PVA, leading to the presence of additional localized states at the interface [57]. The interface effects between CFRO and PVA can create structural disorder, leading to additional localized electronic states [58]. Moreover, the lattice mismatch between the CFRO and PVA can induce strain effects at the interface, altering the density of localized states in the electronic structure [59]. These additional states may broaden the absorption spectrum and increase the Urbach energy, therefore increasing the tail of the absorption edge.
\({U}_{E}\) is exponentially dependent on the absorption coefficient (α) below the optical energy gap. This can be expressed by Urbach’s empirical rule [60]
where \({\alpha }_{o}\)represents a constant, and (\(h\upsilon )\) is the energy of incident photon.
\({U}_{E}\) can be determined by analyzing the reciprocal of the slope of the linear plot of ln (α) versus the photon energy (\(h\upsilon\)) as shown in Fig. 9c, d). A linear fit is applied to the first absorption peak below the optical energy gap, resulting in a slope value of approximately 0.122 eV, and 0.149 eV for CFRO, and CFRO/PVA, respectively. The EU of CFRO has a lower value compared to CFRO/PVA which is the reverse behavior of the \({E}_{g}\).
The increase in UE after coating (CFRO/PVA) typically suggests an increase in the density or distribution of localized electronic states within the band gap. It correlates with the increase in absorbance, indicating a broader distribution of localized states within the absorption spectrum. Thus, the samples tend to absorb a wider range of wavelengths or energies, resulting in higher absorbance values. Higher values of Urbach energy caused the samples to be used as photovoltaic devices and wireless sensors [61].
The refractive index (\(n\)) and the extinction coefficient (\(k\)) are significant parameters that convey serious data about optical materials. The “\(n\)” is regarded as a complex quantity, and it gives precise information on the polarization state due to the electromagnetic field effect of incident light. It can be calculated from the reflectance (R) using the following formula [62]
Figure 10a illustrates the variation of (n) with the (λ) for CFRO, and CFRO/PVA NPs. All values of (n) are remarkably close to each other in the UV–VIS region. The decrease in the values of (n) with increasing λ up to 500 nm is primarily due to the dispersion phenomenon in materials, which can be related to the decreasing polarizability of the investigated samples. The CFRO sample exhibits a maximum refractive index of 2.4 at a wavelength of 720 nm. However, after coating with PVA, the refractive index decreases to 2.2. Both values fall within the appropriate range for integrated optics applications [63].
On the other hand, the “\(k\)” quantifies the amount of light that is scattered and absorbed per unit volume, representing the loss of light in each material. The values of \(k\) \(\left(k=\alpha\lambda/4\pi\right)\) increase with increasing λ, as shown in Fig. 10b, which can be attributed to a significant increase in the absorption in the UV region [62]. The higher values of “\(k\)” for CFRO/PVA can be attributed to the higher absorbance spectrum.
The optical conductivity, \({\sigma }_{Opt}\) is one of the powerful and important tools for investigating the electronic states in materials and can be obtained via (\(\alpha\)) and (\(n\)) [64, 65]
where c is the velocity of light. The variation of the \({\sigma }_{Opt}\)versus the photon energy \(\left(h\upsilon \right)\) for CFRO, and CFRO/PVA NPs is shown in Fig. 11. One can observe that the values of \({\sigma }_{Opt}\) of coated CFRO/PVA are much higher compared to uncoated samples, which may be attributed to the increased photo response of the sample after coating. The higher values of \({\sigma }_{Opt}\) for CFRO/PVA at higher photon energies are due to the increase in the number of excited electrons [65].
Because it is a large and sensitive aspect of creating spectral dispersion devices, dispersion energy plays a crucial role in optical materials. The “n” is linked to two important parameters. The first one is the dispersion energy, \({E}_{d}\), which reflects the average strength of inter-band optical transitions and is associated with the changes in the structural order of the prepared samples. It is associated with the degree of ionic character, the valence of the negatively charged ions, and the number of atoms surrounding them in the given samples. The second parameter is the effective single oscillator energy, \({E}_{0}\), which indicates the average excitation energy necessary for electronic transitions.
The dispersal of refractive index (n) is conducted using the concept of a single oscillator model and represented by the Wimple and DiDomenico (WDD) equation, which it can be expressed in the following relation [66, 67]:
Using Fig. 12a, b, \({E}_{d}\) and \({E}_{o}\) are figured out from the slope and the intercept points of the lines represent the relation between \({({n}^{2}-1)}^{-1}\) versus \({\left(h\upsilon \right)}^{2}\). The obtained data is tabulated in Table 3.
The average gap, \({E}_{o}\), gives quantitative information on the overall band structure of the core-shell prepared samples. This is significantly distinct from the information provided by the optical energy gap (\({E}_{g}\)) value, which explores the optical characteristics of the material near the band edges. Localized states near the \(CB.\) or the \(VB.\) “tail states” may have a strong effect on the optical absorption and thus decrease the optical gap. Whereas if they have a small polarizability, they will result in a small effect on the “\(n\)”: such tail states increase the “\({E}_{U}\)” but have an insignificant effect on the average gap \({E}_{o}\) [11].
By using the WDD relationship, the analysis of dispersion energy parameters also provides more valuable information. Moreover, from this analysis, it is possible to deduce four additional parameters, namely the static refractive index (\({n}_{o})\), the dielectric constant of the lattice \({(\epsilon }_{L})\) and the moments of the optical spectra (\({M}_{-1}\) and \({M}_{-3}\)). The equations that describe the relationship and calculation of these additional parameters are given by [67]:
The optical parameters are estimated and recorded in Table 3. It is obvious that, the values of \({n}_{o}\) and \({\epsilon }_{L}\) increase after being coated with PVA. Additionally, the value of the moment (\({M}_{-1}\)) is increased after coating, while the value of the moment (\({M}_{-3}\)) is decreased.
4 Conclusion
CFRO and CFRO/PVA NPs were effectively produced via the processes of citrate-combustion and ultra-sonication techniques, respectively, in a single phase orthorhombic structure, which is confirmed using XRD. The mean crystalline sizes of the samples are in the range of 20 nm. The experimental lattice parameters decreased while the density increased following the coating of the polymeric shell. HR-TEM microscopic images indicate that the particles have sizes between 40 and 45 nm. EDX analysis indicated that the nanoparticles were successfully synthesized without any impurities. The presence of the core CFRO, and the polymeric shell PVA was confirmed by FT-IR. The increase in ε’ with temperature is due to the interfacial polarization. The difference in the values of ε’ of the samples was attributed to the differences in ionization energies between CFRO and polymers. All samples display anti-ferromagnetic properties, and the values of magnetic parameters decreased after coating by polymeric shell. The values of SF-D and the coercive field of the samples are ideal for achieving high recording performance. The coating by PVA led to a decrease in the optical band gap (Eg), and an increase in the Urbach energy. CFRO/PVA exhibits lower values of refractive index and higher values of extinction coefficient compared to CFRO. The values of \({n}_{o}\), \({\epsilon }_{L}\) and \({M}_{-1}\) increased after coating, while the value of \({M}_{-3}\) decreased.
References
M. Bañobre-López, A. Teijeiro, J. Rivas, Rep. Pract. Oncol. Radiother. 18, 397–400 (2013)
S. Mornet, S. Vasseur, F. Grasset, E. Duguet, J. Mater. Chem. 14, 2161–2161 (2004)
C. Cannas, A. Ardu, D. Peddis, C. Sangregorio, G. Piccaluga, A. Musinu, J. Colloid Interface Sci. 343, 415–422 (2010)
T.E. Torres, A.G. Roca, M.P. Morales, A. Ibarra, C. Marquina, M.R. Ibarra, G.F. Goya, J. Phys.: Conf. Series 200, 072101–072101 (2010)
L. Hualiang, G. Ji, H. Zhang, Y. Du, RSC Adv. 5(33), (2015). https://doi.org/10.1039/C5RA00598A
Y. El-Batawy, M.J. Deen, N. Das, J. Lightwave Technol. 21, 2031–2043 (2003)
A.R. Chavan, P.P. Khirade, S.B. Somvanshi, S.V. Mukhamale, K.M. Jadhav, J. Nanostruct. Chem. 11, 469–481 (2021)
M. George, T.L. Ajeesha, A. Manikandan, A. Anantharaman, R.S. Jansi, E.R. Kumar, Y. Slimani, M.A. Almessiere, A. Baykal, J. Phys. Chem. Solids 153, 110010 (2021)
A.D. Korkmaz, S. Güner, Y. Slimani, H. Gungunes, M. Amir, A. Manikandan, A. Baykal, J. Supercond. Novel Magn. 32, 1057–1065 (2018)
T. Ajeesha, A.A.M. George, A. Manikandan, J.A. Mary, Y. Slimani, M.A. Almessiere, A. Baykal, Phys. B: Condens. Matter 606, 412660 (2021)
E.E. Ateia, Y.A. Saeid, M.K. Abdelmaksoud, J. Supercond. Novel Magn. 36, 1011–1024 (2023)
I. Bibi, M. Muneer, M. Iqbal, N. Alwadai, A.H. Almuqrin, A.S. Altowyan, F.H. Alshammari, A.S. Almuslem, Y. Slimani, Ceram. Int. 47, 31518–31526 (2021)
M. Aamir, I. Bibi, S. Ata, F. Majid, N. Alwadai, A.H. Almuqrin, H. Albalawi, Y. Slimani, M. Bashir, M. Iqbal, Res. Phys. 23, 104023 (2021)
S. Noreen, K. Khalid, M. Iqbal, H.B. Baghdadi, N. Nisar, U.H. Siddiqua, J. Nisar, Y. Slimani, M.I. Khan, A. Nazir, Environ. Technol. Innov. 22, 101430 (2021)
O. Masala, D. Hoffman, N. Sundaram, K. Page, T. Proffen, G. Lawes, R. Seshadri, Solid State Sci. 8, 1015–1022 (2006)
M. Kurian, S. Thankachan, Open Ceram. 8, 100179 (2021)
J. Ma, B. Zhao, H. Xiang, F.-Z. Dai, Y. Liu, R. Zhang, Y. Zhou, J. Adv. Ceram. 11, 754–768 (2022)
Y.A. Saeid, E. Ateia, Appl. Phys. A (2022). https://doi.org/10.1007/s00339-022-05718-3
R. Mitchell, M. Welch, A. Chakhmouradian, Mineral. Mag. 81, 411–461 (2017)
A. Goldman, Handbook of modern ferromagnetic materials (Kluwer Academic Publishers, Dordrecht, 1999)
E.E. Ateia, M.K. Abdelamksoud, M.M. Arman, Rania Ramadan, A.S. Shafaay, Appl. Phys. A, 125, 516 (2019). https://doi.org/10.1007/s00339-019-2815-2
A.C. Gandhi, R. Das, F.-C. Chou, J.G. Lin, J. Phys.: Condens. Matter. 29, 175802–175802 (2017)
A.B. Kadam, V.K. Mande, S.B. Kadam, R.H. Kadam, S.E. Shirsath, R.B. Borade, J. Alloys Compd. 840, 155669 (2020)
A. Wahba, M. Mohamed, J. Magn. Magn. Mater. 378, 246–252 (2015). https://doi.org/10.1016/j.jmmm.2014.10.164
B. Post, X-Ray Spectrom. 4, A18–A18 (1975)
E. Ateia, K. Meleka, F. Ghobrial, Appl. Phys. A (2021). https://doi.org/10.1007/s00339-021-04974-z
E.E. Ateia, A.T. Mohamed, J. Mater. Sci.: Mater. Electron. 31, 19355–19365 (2020)
M. Gurusiddesh, B.J. Madhu ·, G.J. Shankaramurthy, Structural, dielectric, magnetic and electromagnetic interference shielding investigations of polyaniline decorated Co0.5Ni0.5Fe2O4 nanoferrites. J. Mater. Sci.: Mater. Electron. 29, 3502–3509 (2018)
F.H. Martins, V. Pilati, F.L.O. Paula, R.C. Gomes, R. Perzynski, J. Depeyrot, Mater. Res. (2022). https://doi.org/10.1590/1980-5373-MR-2021-0596
D.O. Morais, A. Pancotti, G.S. de Souza, M.V. Saivish, A. Braoios, M.L. Moreli, M.V.B. Souza, V.G. da Costa, J. Wang, J. Mater. Sci. Mater. Med. 32, 101 (2021)
G. Barmparis, Z. Łodziana, N. López, I. Remediakis, Beilstein J. Nanotechnol. 6, 361–368 (2015)
M. Naseri, E. Naderi, A.R. Sadrolhosseini, Fibers Polym. 17, 1667–1674 (2016)
R. Sagayaraj, S. Aravazhi, P. Praveen, G. Chandrasekaran, J. Mater. Sci.: Mater. Electron. 29, 2151–2158 (2018)
C.P. Smyth, Dielectric behavior and structure: dielectric constant and loss, dipole moment and molecular structure (McGraw-Hill, New York, 1995)
A.K. Jonscher, Dielectric relaxation in solids (Chelsea Dielectrics Press, London, 1983)
S.R. Elliott, Adv. Phys. 36, 135–217 (1987)
L. Singh, U.S. Rai, K. Mandal, B.C. Sin, S.-I. Lee, Y. Lee, Ceram. Int. 40, 10073–10083 (2014)
S.A. Mazen, S.F. Mansour, E. Dhahri, H.M. Zaki, T.A. Elmosalami, J. Alloys Compd. 470, 294–300 (2009)
M. Samet, A. Kallel, A. Serghei, J. Compos. Mater. 56, 002199832210906 (2022)
D.A. Johnson, P.G. Nelson, J. Phys. Chem. Ref. Data 46, 013109 (2017)
E.E. Ateia, M.A. Ahmed, R.M. Ghouniem, Solid State Sci. 31, 1–8 (2014). https://doi.org/10.1016/j.solidstatescience.2014.03.002
G. Lal, K. Punia, S.N. Dolia, P.A. Alvi, S. Dalela, S. Kumar, Ceram. Int. 45, 5837–5847 (2019)
H. Li, C.-H. Ra, G. Zhang, W. Yoo, K.-W. Lee, J.-D. Kim, J. Korean Phys. Soc. 54, 1096–1099 (2009)
M.S. Khandekar, R.C. Kambale, J.Y. Patil, Y.D. Kolekar, S.S. Suryavanshi, J Alloys Compod. 509(5), 1861–1865 (2011)
S. Mahalakshmi, K. SrinivasaManja, S. Nithiyanantham, J. Supercond. Novel Magn. 27, 2083–2088 (2014)
R.M. Kershi, F.M. Ali, M.A. Sayed, J. Adv. Ceram. 7, 218–228 (2018)
M.A. Ahmed, N.G. Imam, M.K. Abdelmaksoud, Y.A. Saeid, J. Rare Earths 33, 965–971 (2015)
R.K. Kotnala, Jyoti Shah, Handbook of magnetic materials (Elsevier, Amsterdam, 2015)
A. Omelyanchik, S. Villa, G. Singh, V. Rodionova, S. Laureti, F. Canepa, D. Peddis, Magnetochemistry 7, 146 (2021)
E.E. Ateia, K. Elsayed, D.E. El-Nashar, Appl. Phys. A 129, 118 (2023)
E.E. Ateia, M. Farag, Appl. Phys. A 125, 324 (2019)
E.E. Ateia, M.A. Ateia, M.M. Arman, J. Mater. Sci.: Mater. Electron. 33, 8958–8969 (2022)
R. Badry, M.A. Hegazy, I.S. Yahia, H. Elhaes, H.Y. Zahran, A.I. Abdel-Salam, H. Matar, M.A. Ibrahim, Egypt J. Chem 65, 335–342 (2022)
Z.K. Heiba, M.B. Mohamed, N.Y. Mostafa, A.M. ElNaggar, J. Inorg. Organomet. Polym. Mater. 30, 1898–1906 (2020)
D.E. Abulyazied, H.M. Abomostafa, G.M. El Komy, J. Inorg. Organomet. Polym. Mater. 30, 2335–2346 (2020)
A. Aridi, R. Awad, A. Khalaf, Appl. Phys. A 127, 206 (2021)
F. Poulsen, T. Hansen, J. Phys. Chem. C (2017). https://doi.org/10.1021/acs.jpcc.7b01792
I. Studenyak, M. Kranjčec, M. Kurik, Int. J. Opt. Appl. 4, 76–83 (2014)
J.T.L. Gamler, A. Leonardi, X. Sang, K.M. Koczkur, R.R. Unocic, M. Engel, S.E. Skrabalak, Nanoscale Adv. 2, 1105–1114 (2020)
A.S. Hassanien, A.A. Akl, Superlattices Microstruct. 89, 153–169 (2016)
B. Zhao, M. Abdi-Jalebi, M. Tabachnyk, H. Glass, V.S. Kamboj, W. Nie, A.J. Pearson, Y. Puttisong, K.C. Gödel, H.E. Beere, D.A. Ritchie, A.D. Mohite, S.E. Dutton, R.H. Friend, A. Sadhanala, Adv. Mater. 29, 1604744 (2017)
N.S. Kozlova, O.A. Buzanov, V.M. Kasimova, A.P. Kozlova, E.V. Zabelina, Mod. Electron. Mater. 4, 7–12 (2018)
D. Erdem, N.S. Bingham, F.J. Heiligtag, N. Pilet, P. Warnicke, L.J. Heyderman, M. Niederberger, Adv. Funct. Mater. 26, 1954–1963 (2016)
M. Sultan, R. Singh, J. Phys. D 42, 115306 (2009)
A. Elbadawi, E. Mustafa, N. Elthair, Open J. Appl. Sci. 10, 551–560 (2020)
A. Abdel Moez, S.A. Gad, Egypt. J. Chem. 64, 1109–1115 (2021)
A.S. Hassanien, R. Neffati, K.A. Aly, Optik 212, 164681 (2020)
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). The authors have not disclosed any funding.
Author information
Authors and Affiliations
Contributions
EEA involved in the development of the concept, validation process, preparation of the first draft, review and editing of the final manuscript, creation of visual aids, and provided oversight. YAS participated in material preparation, data collection and analysis, formal analysis, investigation, selection of optimal material parameters, development of methodology, writing the initial draft, validation, visualization, as well as review and editing.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ateia, E.E., Saeid, Y.A. Characterization of Core–Shell CaFe1.925Sm0.05Gd0.025O4 @ Polymer from Synthesis to Applications. J Inorg Organomet Polym 34, 118–130 (2024). https://doi.org/10.1007/s10904-023-02799-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10904-023-02799-2