Abstract
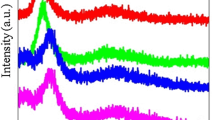
Synthesis of tellurite borate (35-x) TeO2 + 35 B2O3 + 20 Bi2O3 + 10 Li2O + x CeO2 and x = 0, 0.5, 1.5, 3 mol% glasses have been achieved by using the melt quenching technique. Density, X-ray diffraction (XRD), Raman spectroscopy measurements and UV–Vis analysis were measured. Dielectric measurements were recorded in the frequency range of 100 Hz–100 kHz at room temperature. XRD patterns of all the investigated glass samples doped with CeO2 are completely amorphous. Densities of the prepared glasses increased from 4.80 to 4.89 g/cm3 and molar volumes decreased from 36.74 to 36.14 cm3/mole. Raman spectroscopy reveals that the Raman intensity increases with increasing substitution of CeO2 by TeO2. UV–Vis analysis reveals that a higher CeO2 substitution ratio increased the absorbance and optical properties of produced glass samples. The CeO2 content of tellurium borate glass samples were found to affect all of the optical characteristics. With increasing CeO2 concentration, the optical energy gap decreases \((E_{g}^{opt}\)) from 2.77 to 1.96 eV for direct allowed transitions and from 2.45 to 1.82 eV for indirect allowed transitions. It has also been studied how electrical conductivity and dielectric properties change with frequency and composition. Values ε′, ε″ and σac of sample with x = 0 are greater than those values of with x = 3. The variation of the imaginary part of the electric modulus (M″) versus frequency (F) plot curve is asymmetric of non-Lorentzian type. A shift of the frequency peak towards the higher frequency region was observed by increasing CeO2 mol%.



















Similar content being viewed by others
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Y. Morova et al., Tunable continuous-wave laser operation of Tm3+ ion doped tellurite glass near 2 μm. J. Lumin. 252, 119318 (2022)
P. Patra, K. Annapurna, Transparent tellurite glass-ceramics for photonics applications: a comprehensive review on crystalline phases and crystallization mechanisms. Prog. Mater. Sci 125, 100890 (2022)
M.A. Sidkey, R.A. El Mallawany, A.A. Abousehly, Y.B. Saddeek, Relaxation of longitudinal ultrasonic waves in some tellurite glasses. Mater. Chem. Phys. 74(2), 222–229 (2002)
R.N. Hampton, W. Hong, G.A. Saunders, R. El-Mallawany, The electrical conductivity of pure and binary TeO2 glasses. J. Non-Cryst. Solids 94(3), 307–314 (1987)
R.N. Hampton, W. Hong, G. Saunders, R. El-Mallawany, Dielectric properties of tellurite glass. Phys. Chem. Glasses 29(3), 100–105 (1988)
A. Abdel-Kader, A.A. Higazy, R. El-Mallawany, M. Elkholy, The effect of gamma irradiation on the electrical conductivity of TeO2-P2O5 and Bi2O3-TeO2-P2O5 glasses. Radiat. Effects Defects Solids 124, 401–407 (1992)
C. Li, L. Zhu, D. Zhao, J. Li, Y. Zhou, Broadband NIR radiative transitions in Er3+/Tm3+ co-doping tellurite glass material. Mater. Res. Bull. 158, 112042 (2023)
R. El-Mallawany, A. Abd El-Moneim, Comparison between the elastic moduli of tellurite and phosphate glasses. Phys. Status Solidi A 166, 829–834 (1998)
J. He, L. Chen, H. Li, J. Niu, Y. Ma, Novel 3.1 µm and enhanced 2.7 µm emissions in Er3+ doped fluorotellurite glasses ceramic. J. Alloys Compds. 895, 162606 (2022)
R. Boda, M.D. Shareefuddin, M.N. Chary, R. Sayanna, FTIR and optical properties of europium doped lithium zinc bismuth borate glasses. Mater. Today 3, 1914–1922 (2016)
N. Gupta, A. Kaur, A. Khanna, F. Gonzàlez, C. Pesquera, R. Iordanova, B. Chen, Structure-property correlations in TiO2-Bi2O3- B2O3-TeO2 glasses. J. Non-Cryst. Solids 470, 168–177 (2017)
T.M. Machado, L.P.F. Peixoto, G.F.S. Andrade, M.A.P. Silva, Copper nanoparticles–containing tellurite glasses: an efficient SERS substrate. Mater. Chem. Phys. 278, 125597 (2022)
R. El-Mallawany, M. Sidkey, A. Khafagy, H. Afifi, Elastic constants of semiconducting tellurite glasses. Mater. Chem. Phys. 37, 295–298 (1994)
I.Z. Hager, R. El-Mallawany, M. Poulain, Infrared and Raman spectra of new molybdenum and tungsten oxyfluoride glasses. J. Mater. Sci. 34(21), 5163–5168 (1999)
F. Aouaini, A. Maaoui, N.B. Mohamed, M.M. Alanazi, L.A. El Maati, Visible to infrared down conversion of Er3+ doped tellurite glass for luminescent solar converters. J. Alloys Compd. 894, 162506 (2022)
M.P. Belançon, M. Sandrini, H.S. Muniza, L.S. Herculano, G.V.B. Lukasievicz, E.L. Savic, O.A. Capeloto, L.C. Malacarne, N.G.C. Astrath, M.L. Baesso, G.J. Schiavond, A.A. Silva Juniord, J.D. Marconie, Float, borosilicate and tellurites as cover glasses in Si photovoltaics: Optical properties and performances under sunlight. J. Phys. Chem. Solids 161, 110396 (2022)
I. Ferodolin, A. Awang, S.K. Ghoshal, A. Samavati, Ch.F. Pien, J. Dayoud, Plasmonic effect of bimetallic TiO2/Al2O3 nanoparticles in tellurite glass for surface-enhanced Raman scattering applications. J. Lumin. 241, 118488 (2022)
T.O. Sales, C. Jacinto, W.F. Silva, R. Antunes, D.T. Dias, A. Gonçalves, R. El-Mallawany, N.G.C. Astrath, A. Novatski, White light source and optical thermometry based on zinc-tellurite glass tri-doped with Tm3+/Er3+/Sm3+. J. Alloys Compd. 899, 163305 (2022)
S.F. Hosseini, D. Souri, A.N. Emamzadeh, Functional thermal stable samples: non-isothermal calorimetric analysis of MoO3–V2O5–TeO2 oxide glasses. J. Inorg. Organomet. Polym. Mater. 31, 2877–2890 (2021)
A. Aşkın, M.I. Sayyed, A. Sharma, M. Dal, R. El-Mallawany, M.R. Kaçal, Investigation of the gamma ray shielding parameters of (100–x) [0.5 Li2O–0.1 B2O3–0.4 P2O5]-xTeO2 glasses using Geant4 and FLUKA codes. J. Non-Cryst. Solids 521, 119489 (2019)
G.P. Singh, P. Kaur, S. Kaur, D.P. Singh, Investigation of structural, physical and optical properties of CeO2–Bi2O3– B2O3 glasses. Phys. B 407, 4168–4172 (2012)
H.A. Abo-Mosallam, S.E. Ibrahim, The impact of V2O5 and SnO2 on structure, physical and electrical properties of lithium copper fluorophosphate glasses. J. Non Cryst. Solids 592, 121745 (2022)
S.R. Rejisha, P.S. Anjana, N. Gopakumar, Effect of cerium(IV) oxide on the optical and dielectric properties of strontium bismuth borate glasses. J. Mater. Sci. 27, 5475–5482 (2016)
M. Ceriotti, F. Pietrucci, M. Bernasconi, Ab initio, study of the vibrational properties of crystalline TeO2: α, β, and γ phases. Phys. Rev. B 73, 104304 (2006)
A. Kaur, A. Khanna, F. González, C. Pesquera, B. Chen, Structural, optical, dielectric and thermal properties of molybdenum tellurite and borotellurite glasses. J. Non- Cryst. Solids 444, 1–10 (2016)
J.C. Sabadel, P. Armand, D. Cachau-Herreillat, P. Baldeck, O. Doclot, A. Ibanez, E. Philippot, Structural and nonlinear optical characterizations of tellurium oxidebased glasses: TeO2–BaO–TiO2. J. Solid State Chem. 132, 411–419 (1997)
R.S. Kundu, S. Dhankhar, R. Punia, K. Nanda, N. Kishore, Bismuth modified physical, structural and optical properties of mid-IR transparent zinc boro-tellurite glasses. J. Alloys Compd. 587, 66–73 (2014)
P. Colomban, A. Slodczyk, Raman intensity: an important tool to study the structure and phase transitions of amorphous/crystalline materials. Opt. Mater. 31, 1759–1763 (2009)
E.A. Davis, N.F. Mott, Conduction in non-crystalline systems V. Conductivity, optical absorption and photoconductivity in amorphous semiconductors. Philos. Mag. (1970). https://doi.org/10.1080/14786437008221061
A.S. Abouhaswa, Y.S. Rammah, S.E. Ibrahim, A.A. El-Hamalawy, Structural, optical, and electrical characterization of borate glasses doped with SnO2. J. Non Cryst. Solids (2018). https://doi.org/10.1016/j.jnoncrysol.2018.04.051
P. Chimalawong, J. Kaewkhao, C. Kedkaew, P. Limsuwan, Optical and electronic polarizability investigation of Nd3+-doped soda-lime silicate glasses. J. Phys. Chem. Solids. 71, 965–970 (2010)
R.S. Gedam, D.D. Ramteke, Influence of CeO2 addition on the electrical and optical properties of lithium borate glasses. J. Phys. Chem. Solids 74, 1399–1402 (2013)
F. Urbach, The long-wavelength edge of photographic sensitivity and of the electronic absorption of solids. Phys. Rev. (1953). https://doi.org/10.1103/PhysRev.92.1324
F. Abeles, Opt Prop. Solids (North-Holland Publ Company, London, 1972)
S.H. Wemple, M. DiDomenico, Behavior of the electronic dielectric constant in covalent and ionic materials. Phys. Rev. B (1971). https://doi.org/10.1103/PhysRevB.3.1338
N. Gupta, A. Kaur, A. Khanna, F. Gonzàlez, C. Pesquer, R. Iordanova, B. Chen, Structure-property correlations in TiO2-Bi2O3-B2O3-TeO2 glasses. J. Non Cryst. Solids 470, 168–177 (2017)
A. Kaur, A. Khanna, P.S.R. Krishna, A.B. Shinde, M. González-Barriuso, F. González, B. Chen, Structure of copper tellurite and borotellurite glasses by neutron diffraction, Raman, 11B MASNMR and FTIR spectroscopy. Phys. Chem. Glasses 61, 27–39 (2020)
R.M.M. Morsi, S.I.A. El-Ghany, M.M. Morsi, Electrical properties of silicate glasses of low level gadolinium oxide doping including dielectric and infrared measures. J. Mater. Sci. 26, 1419–1426 (2015). https://doi.org/10.1007/s10854-014-2556-0
A.M. Nawar, H.M. Abd El-Khalek, M.M. El-Nahass, Dielectric and electric modulus studies on Ni (II) tetraphenyl porphyrin thin films. Org. Opto-Elect. 1, 25–38 (2015)
F.S. Howell, R.A. Bose, P.B. Macedo, C.T. Moynihan, Electrical relaxation in a glass-forming molten salt. J. Phys. Chem. 78, 639–648 (1974)
F. Yakuphanoglu, I.S. Yahia, B.F. Senkal, G.B. Sakr, W.A. Farooq, Impedance spectroscopy properties of polypyrrole doped with boric acid. Synth. Met. 161, 817–822 (2011)
S. Chisca, V.E. Musteata, I. Sava, M. Bruma, Dielectric behavior of some aromatic polyimide films. J. Eur. Polym. 47, 1186–1197 (2011)
S. Kurien, J. Mathew, S. Sebastian, S.N. Potty, K.C. George, Dielectric behavior and ac electrical conductivity of nanocrystalline nickel aluminate. Mater. Chem. Phys. 98, 470–476 (2006)
D.K. Mahato, A. Dutta, T.P. Sinha, Dielectric relaxation and ac conductivity of double perovskite oxide Ho2ZnZrO6. Physica B 406, 2703–2708 (2011)
M. Eltabey et al., Structural, electrical and magnetic properties of high iron content sodium borosilicate glass. J. Appl. Phys. 8, 95–102 (2016)
K. Majhi, K.B.R. Varma, K.J. Rao, Possible mechanism of charge transport and dielectric relaxation in SrO-Bi2O3-B2O3 glass. J. Appl. Phys. 106, 084106 (2009)
M.T. Ahmed, H. Elhendawy, Z.M. Elqahtani, W.B. Elsharkawy, M.A. Azzam, T. Fahmy, Electric modulus and scaling behaviour of Chitosan/PVA biopolymer blend Egypt. J. Chem. 65, 459–471 (2022)
M. Boora, S. Malik, V. Kumar, M. Bala, S. Arora, S. Rohilla, A. Kumar, J. Dala, Investigation of structural and impedance spectroscopic properties of borate glasses with high Li+ concentration. Solid State Ionics 368, 115704 (2021)
S. Dahiya, R. Punia, A. Singh, A.S. Maan, S. Murugavel, DC conduction and electric modulus formulation of lithium-doped bismuth zinc vanadate semiconducting glassy system. J. Am. Ceram. Soc. 98, 2776–2783 (2015)
M. Tijaria, Y. Sharma, V. Kumar, S. Dahiya, J. Dalal, Effect of Na2O on physical, structural and electrical properties of borate glasses. Mater. Today 45, 3722–3725 (2021)
Y.J. Wong, J. Hassan, M. Hashim, Dielectric properties impedance analysis and modulus behavior of CaTiO3 ceramic prepared by solid reaction. J. Alloys Compd. 571, 138–144 (2013)
S.B. Aziz, O.G. Abdullah, S.R. Saeed, H.M. Ahmed, Electrical and dielectric properties of copper Ion conducting solid polymer electrolytes based on Chitosan: CBH model for ion transport mechanism. Int. J. Electrochem. Sci. 13, 3812–3826 (2018). https://doi.org/10.20964/2018.04.10
A. Dutta, T.P. Sinha, P. Jena, S. Adak, Ac conductivity and dielectric relaxation in ionically conducting soda-lime-silicate glasses. J. Non-Cryst. Solids 354, 3952–3957 (2008)
S. Bhattacharya, A. Ghosh, Conductivity relaxation in some fast ion-conducting AgI-Ag2O-V2O5 glasses. Solid State Ionics 161, 61–65 (2003)
R.V. Barde, K.R. Nemade, S.A. Waghuley, AC conductivity and dielectric relaxation in V2O5–P2O5–B2O3 glasses. J. Asian Ceram. Soc. 3, 116–122 (2015)
R. El-Mallawany, A.H. El-Sayed, M.M.H.A. El-Gawad, ESR and electrical conductivity studies of (TeO2) 0.95 (CeO2) 0.05 semiconducting glasses. Mater. Chem. Phys. 41(2), 87–91 (1995)
Funding
The authors have not disclosed any funding.
Author information
Authors and Affiliations
Contributions
SEI and ASA performed all the experimental work (Glass samples preparation and its characterization) and prepared manuscript. RE-M helped significantly in the explanation of experimental results and revised manuscript.
Corresponding author
Ethics declarations
Conflict of interest
There is no conflict of interest among the contributing authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ibrahim, S.E., El-Mallawany, R. & Abouhaswa, A.S. Structural, Optical and Dielectric Properties of Tellurite Borate Glasses Doped with Cerium Oxide. J Inorg Organomet Polym 33, 2319–2330 (2023). https://doi.org/10.1007/s10904-023-02682-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10904-023-02682-0