Abstract
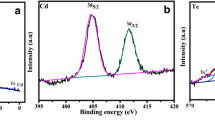
The hydrothermal technique was used to make nickel telluride nanorods (NiTe NRs) utilizing ascorbic acid and cetrimonium bromide (CTAB) as reducing agents. Temperature dependent magnetic study for NiTe NRs shows a ferromagnetism behavior. Under 532 nm laser excitation, the obtained materials had a better optical limiting property, with a two photon absorption coefficient of 6.6 × 10− 10 m/W and an optical limiting of 2.44 J/cm2 at 200 µJ. NiTe NRs modified electrode shows a excellent hydrogen peroxide electrocatalytic activity with reproducibility, repeatability and durability. It displays an outstanding sensitivity of 6.35 µAµM− 1 cm− 2 and a detection limit of 6 nM. In the presence of interfering species such as dopamine, uric acid, ascorbic acid, glucose, and folic acid, the electrode has a high level of selectivity. A real sample analysis for NiTe NRs sensor has been established in human serum and rat brain serum showed good recoveries.









Similar content being viewed by others
Change history
23 May 2023
A Correction to this paper has been published: https://doi.org/10.1007/s10904-023-02697-7
References
Y. Li, J.J. Zhang, J. Xuan, L.P. Jiang, J.J. Zhu, Fabrication of a novel nonenzymatic hydrogen peroxide sensor based on Se/Pt nanocomposites. Electrochem. commun. 12, 777–780 (2010). https://doi.org/10.1016/j.elecom.2010.03.031
K. Fang, Y. Yang, L. Fu, H. Zheng, J. Yuan, L. Niu, Highly selective H2O2 sensor based on 1-D nanoporous Pt@C hybrids with core-shell structure. Sensors Actuators, B Chem. 191, 401–407 (2014). https://doi.org/10.1016/j.snb.2013.09.090
S. Kogularasu, M. Govindasamy, S.M. Chen, M. Akilarasan, V. Mani, 3D graphene oxide-cobalt oxide polyhedrons for highly sensitive non-enzymatic electrochemical determination of hydrogen peroxide. Sensors Actuators B Chem. 253, 773–783 (2017). https://doi.org/10.1016/j.snb.2017.06.172
S. Dong, J. Xi, Y. Wu, H. Liu, C. Fu, H. Liu, F. Xiao, High loading MnO2 nanowires on graphene paper: Facile electrochemical synthesis and use as flexible electrode for tracking hydrogen peroxide secretion in live cells. Anal. Chim. Acta 853, 200–206 (2015). https://doi.org/10.1016/j.aca.2014.08.004
S. Kubendhiran, B. Thirumalraj, S. Chen, C. Karuppiah, Journal of Colloid and Interface Science Electrochemical co-preparation of cobalt sulfide/reduced graphene oxide composite for electrocatalytic activity and determination of H2O2 in biological samples. J. Colloid Interface Sci. 509, 153–162 (2018). https://doi.org/10.1016/j.jcis.2017.08.087
K.J. Babu, A. Zahoor, K.S. Nahm, R. Ramachandran, M.A.J. Rajan, G. Gnana-Kumar, The influences of shape and structure of MnO2 nanomaterials over the non-enzymatic sensing ability of hydrogen peroxide. J. Nanoparticle Res. 16, 2250 (2014). https://doi.org/10.1007/s11051-014-2250-4
T. Zheng, X. Lu, X. Bian, C. Zhang, Y. Xue, X. Jia, C. Wang, Fabrication of ternary CNT/PPy/K xMnO 2 composite nanowires for electrocatalytic applications. Talanta 90, 51–56 (2012). https://doi.org/10.1016/j.talanta.2011.12.066
A.K. Dutta, S. Das, P.K. Samanta, S. Roy, B. Adhikary, P. Biswas, Non-enzymatic amperometric sensing of hydrogen peroxide at a CuS modified electrode for the determination of urine H2O2. Electrochim. Acta. 144, 282–287 (2014). https://doi.org/10.1016/j.electacta.2014.08.051
L. Wan, J. Liu, X.-J. Huang, Novel magnetic nickel telluride nanowires decorated with thorns: synthesis and their intrinsic peroxidase-like activity for detection of glucose. Chem. Commun 50, 13589–13591 (2014). https://doi.org/10.1039/c4cc06684g
M. Manikandan, P.N. Francis, S. Dhanuskodi, N. Maheswari, G. Muralidharan, High performance supercapacitor behavior of hydrothermally synthesized CdTe nanorods. J. Mater. Sci. Mater. Electron. 29, 17397–17404 (2018). https://doi.org/10.1007/s10854-018-9837-y
A. Padmanaban, N. Padmanathan, T. Dhanasekaran, R. Manigandan, S. Srinandhini, P. Sivaprakash, S. Arumugam, V. Narayanan, Hexagonal phase Pt-doped cobalt telluride magnetic semiconductor nanoflakes for electrochemical sensing of dopamine. J. Electroanal. Chem. 877, 114658 (2020). https://doi.org/10.1016/j.jelechem.2020.114658
B. Golrokh-Amin, U. De Silva, J. Masud, M. Nath, Ultrasensitive and highly selective Ni3Te2 as a nonenzymatic glucose sensor at extremely low working potential. ACS Omega 4, 11152–11162 (2019). https://doi.org/10.1021/acsomega.9b01063
B. Fatima, U. Saeed, D. Hussain, S.E.Z. Jawad, H.S. Rafiq, S. Majeed, S. Manzoor, S.Y. Qadir, M.N. Ashiq, M. Najam-ul-Haq, Facile hydrothermal synthesis of NiTe nanorods for non-enzymatic electrochemical sensing of whole blood hemoglobin in pregnant anemic women. Anal. Chim. Acta. 1189, 339204 (2022). https://doi.org/10.1016/j.aca.2021.339204
J.J. Zhang, Y.G. Liu, L.P. Jiang, J.J. Zhu, Synthesis, characterizations of silica-coated gold nanorods and its applications in electroanalysis of hemoglobin. Electrochem. Commun. 10, 355–358 (2008). https://doi.org/10.1016/j.elecom.2007.12.017
Y. Liu, J. Zhang, W. Hou, J.J. Zhu, A Pd/SBA-15 composite: synthesis, characterization and protein biosensing. Nanotechnology 19, 135707 (2008). https://doi.org/10.1088/0957-4484/19/13/135707
K.S. Bhat, H.C. Barshilia, H.S. Nagaraja, Porous nickel telluride nanostructures as bifunctional electrocatalyst towards hydrogen and oxygen evolution reaction. Int. J. Hydrogen Energy. 42, 24645–24655 (2017). https://doi.org/10.1016/j.ijhydene.2017.08.098
Z. Wang, L. Zhang, In situ growth of NiTe nanosheet film on nickel foam as electrocatalyst for oxygen evolution reaction. Electrochem. commun. 88, 29–33 (2018). https://doi.org/10.1016/j.elecom.2018.01.014
S. Pradhan, R. Das, S. Biswas, D.K. Das, R. Bhar, R. Bandyopadhyay, P. Pramanik, Chemical synthesis of nanoparticles of nickel telluride and cobalt telluride and its electrochemical applications for determination of uric acid and adenine. Electrochim Acta. 238, 185–193 (2017). https://doi.org/10.1016/j.electacta.2017.04.023
S.M. Yong, P. Muralidharan, S.H. Jo, D.K. Kim, One-step hydrothermal synthesis of CdTe nanowires with amorphous carbon sheaths. Mater. Lett. 64, 1551–1554 (2010). https://doi.org/10.1016/j.matlet.2010.04.045
M. Manikandan, K. Subramani, M. Sathish, S. Dhanuskodi, NiTe nanorods as electrode material for high performance supercapacitor applications. ChemistrySelect 3, 9034–9040 (2018). https://doi.org/10.1002/slct.201801421
M. Manikandan, K. Subramani, S. Dhanuskodi, M. Sathish, One-pot hydrothermal synthesis of nickel cobalt telluride nanorods for hybrid energy storage systems. Energy Fuels 35, 12527–12537 (2021). https://doi.org/10.1021/acs.energyfuels.1c00351
J. Song, Y. Lin, Y. Zhan, Y. Tian, G. Liu, S. Yu, Superlong high-quality tellurium nanotubes: synthesis, characterization and optical property. Cryst. Growth Des. 8, 1902–1908 (2008). https://doi.org/10.1021/cg701125k
H. Chen, M. Fan, C. Li, G. Tian, C. Lv, D. Chen, K. Shu, J. Jiang, One-pot synthesis of hollow NiSe-CoSe nanoparticles with improved performance for hybrid supercapacitors. J. Power Sources. 329, 314–322 (2016). https://doi.org/10.1016/j.jpowsour.2016.08.097
Y. Lei, N. Miao, J. Zhou, Q.U. Hassan, J. Wang, Novel magnetic properties of CoTe nanorods and diversified CoTe2 nanostructures obtained at different NaOH concentrations. Sci. Technol. Adv. Mater. 18, 325–333 (2017). https://doi.org/10.1080/14686996.2017.1317218
N. Umeyama, M. Tokumoto, S. Yagi, M. Tomura, K. Tokiwa, T. Fujii, R. Toda, N. Miyakawa, S.I. Ikeda, Synthesis and magnetic properties of NiSe, NiTe, CoSe, and CoTe. Jpn. J. Appl. Phys. 51, 053001 (2012). https://doi.org/10.1143/JJAP.51.053001
M. Ragam, G. Kalaiselvan, S. Arumugam, N. Sankar, K. Ramachandran, Room temperature ferromagnetism in MnxZn1−xS (x=0.00–0.07) nanoparticles. J. Alloys Compd. 541, 222–226 (2012). https://doi.org/10.1016/j.jallcom.2012.07.024
A. Seetharaman, D. Sivasubramanian, V. Gandhiraj, V.R. Soma, Tunable nanosecond and femtosecond nonlinear optical properties of C-N-S-doped TiO2 nanoparticles. J. Phys. Chem. C. 121, 24192–24205 (2017). https://doi.org/10.1021/acs.jpcc.7b08778
M. Zhao, R. Peng, Q. Zheng, Q. Wang, M.-J. Chang, Y. Liu, Y.-L. Song, H.-L. Zhang, Broadband optical limiting response of a graphene–PbS nanohybrid. Nanoscale 7, 9268–9274 (2015). https://doi.org/10.1039/C5NR01088H
C.S. Suchand-Sandeep, A.K. Samal, T. Pradeep, R. Philip, Optical limiting properties of Te and Ag2Te nanowires. Chem. Phys. Lett. 485, 326–330 (2010). https://doi.org/10.1016/j.cplett.2009.12.065
K. Sridharan, V. Tamilselvan, D. Yuvaraj, K. Narasimha Rao, R. Philip, Synthesis and nonlinear optical properties of lead telluride nanorods. Opt. Mater. 34, 639–645 (2012). https://doi.org/10.1016/j.optmat.2011.09.009
A. Das, S. Ratha, R.K. Yadav, A. Mondal, C.S. Rout, K.V. Adarsh, Strong third-order nonlinear response and optical limiting of α-NiMoO4 nanoparticles. J Appl. Phys. 122, 013107 (2017). https://doi.org/10.1063/1.4992806
M. Govindasamy, V. Mani, S.M. Chen, R. Karthik, K. Manibalan, R. Umamaheswari, MoS2 flowers grown on graphene/carbon nanotubes: A versatile substrate for electrochemical determination of hydrogen peroxide. Int. J. Electrochem. Sci. 11, 2954–2961 (2016). https://doi.org/10.20964/110402954
M. Manikandan, S. Dhanuskodi, N. Maheswari, G. Muralidharan, C. Revathi, R.T. Rajendra Kumar, G. Mohan-Rao, High performance supercapacitor and non-enzymatic hydrogen peroxide sensor based on tellurium nanoparticles. Sens. Bio-Sensing Res. 13, 40–48 (2017). https://doi.org/10.1016/j.sbsr.2017.02.001
M.R. Guascito, D. Chirizzi, C. Malitesta, T. Siciliano, A. Tepore, Te oxide nanowires as advanced materials for amperometric nonenzymatic hydrogen peroxide sensing. Talanta 115, 863–869 (2013). https://doi.org/10.1016/j.talanta.2013.06.032
M.R. Guascito, D. Chirizzi, C. Malitesta, E. Mazzotta, M. Siciliano, T. Siciliano, A. Tepore, A. Turco, Low-potential sensitive H2O2 detection based on composite micro tubular Te adsorbed on platinum electrode. Biosens. Bioelectron. 26, 3562–3569 (2011). https://doi.org/10.1016/j.bios.2011.02.002
M. Mayilmurugan, G. Rajamanickam, R. Perumalsamy, D. Sivasubramanian, Nickel cobalt telluride nanorods for sensing the hydrogen peroxide in living cells. ACS Omega 7, 14556–14561 (2022). https://doi.org/10.1021/acsomega.1c06007
Acknowledgment
This work was funded by the Researchers Supporting Project Number (RSP-2021/265) King Saud University, Riyadh, Saudi Arabia.
Author information
Authors and Affiliations
Contributions
Hydrothermal synthesis of NiTe Nanorods. Ferromagnetic behavior. Optical limiting of 2.44 J cm−2 at 200 µJ. Excellent sensitivity 6.35 µA µM−1 cm−2 with detection limit of 6 nM. The sensor exhibit stability, repeatability and reproducibility.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing financial interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: The original version of this article unfortunately contained a mistake. The project number in the Acknowledgments section was incorrect. The correct project number is RSP-2021/265. The original article has been corrected.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Manikandan, M., Manikandan, E., Alshgari, R.A. et al. NiTe Magnetic Semiconductor Nanorods for Optical Limiting and Hydrogen Peroxide Sensor. J Inorg Organomet Polym 33, 1538–1547 (2023). https://doi.org/10.1007/s10904-023-02565-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10904-023-02565-4