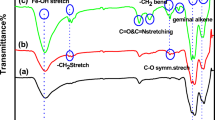
Abstract
Enzymes are extensively used as catalyst in several fields of production such as chemistry, and pharmaceuticals owing to their selectivity, efficiency and environmentally friendliness. However, their applications are often hindered due to their insufficient stability and difficulties in re-use. As a member of porous crystalline materials, metal organic frameworks are a promising enzyme carrier due to their multi-functional pore surfaces and robustness in variety of harsh conditions. In this study, the horseradish peroxidase (HRP) enzyme was immobilized onto UiO-66-NH2 (Universitetet i Oslo) by a facile incubation method at the room temperature to improve the stability and reusability of enzyme. The prepared HRP@UiO-66-NH2 bio-composite was characterized by using FT-IR, XRD and SEM. The crystal structure of MOF was well-preserved after enzyme immobilization. A colorimetric assay for enzyme activity after released from UiO-66-NH2 has been employed based on the catalytic oxidation of phenol coupled with 4-aminoantipyrine. The robustness and activity of immobilized enzyme after released from UiO-66-NH2 were investigated by biodegradation of methyl orange (MO) and methylene blue (MB) with several parameters such as pH, temperature, the dosage of H2O2 and the dye concentration with comparison to its free form. The optimum condition for dye degradation was obtained at basic conditions. The immobilized enzyme maintained its activity at elevated temperature while free enzyme lost its activity at the same conditions, attributed to the armoring effect of UiO-66-NH2. According to the results of studied various parameters, MO and MB were biodegraded to 60% and 45%, respectively, within 60 min with the optimum conditions at pH 9 and 50 °C at a H2O2 dosage of 3%. The superior pH tolerance and stability suggest potential of UiO-66-NH2 immobilized peroxidase enzyme in industrial applications.





Similar content being viewed by others
References
F.U. Hartl, A. Bracher, M. Hayer-Hartl, Molecular chaperones in protein folding and proteostasis. Nature 475, 324–332 (2011)
J. Cui, S. Ren, B. Sun, S. Jia, Optimization protocols and improved strategies for metal-organic frameworks for immobilizing enzymes: Current development and future challenges. Coord. Chem. Rev. 370, 22–41 (2018)
N.J. Turner, Directed evolution drives the next generation of biocatalysts. Nat. Chem. Biol. 5, 567–573 (2009)
J.L. Porter, R.A. Rusli, D.L. Ollis, Directed evolution of enzymes for industrial biocatalysis. ChemBioChem 17, 197–203 (2016)
R.J. Drout, L. Robison, O.K. Farha, Catalytic applications of enzymes encapsulated in metal–organic frameworks. Coord. Chem. Rev. 381, 151–160 (2019)
U. Bornscheuer, G. Huisman, R.J. Kazlauskas, S. Lutz, J. Moore, K. Robins, Engineering the third wave of biocatalysis. Nature 485, 185–194 (2012)
T. Heimbach, D.-M. Oh, L.Y. Li, N.R. Rodrı́guez-Hornedo, G. Garcia, D. Fleisher, Enzyme-mediated precipitation of parent drugs from their phosphate prodrugs. Int. J. Pharm. 261, 81–92 (2003)
B. Schulze, M.G. Wubbolts, Biocatalysis for industrial production of fine chemicals. Curr. Opin. Biotechnol. 10, 609–615 (1999)
S. Luetz, L. Giver, J. Lalonde, Engineered enzymes for chemical production. Biotechnol. Bioeng. 101, 647–653 (2008)
C. Mateo, J.M. Palomo, G. Fernandez-Lorente, J.M. Guisan, R. Fernandez-Lafuente, Improvement of enzyme activity, stability and selectivity via immobilization techniques. Enzyme Microb. Technol. 40, 1451–1463 (2007)
U. Hanefeld, L. Gardossi, E. Magner, Understanding enzyme immobilisation. Chem. Soc. Rev. 38, 453–468 (2009)
P.V. Iyer, L. Ananthanarayan, Enzyme stability and stabilization—aqueous and non-aqueous environment. Process Biochem. 43, 1019–1032 (2008)
C.S. Thomas, M.J. Glassman, B.D. Olsen, Solid-state nanostructured materials from self-assembly of a globular protein–polymer diblock copolymer. ACS Nano 5, 5697–5707 (2011)
A. Bhunia, S. Durani, P.P. Wangikar, Horseradish peroxidase catalyzed degradation of industrially important dyes. Biotechnol. Bioeng. 72, 562–567 (2001)
A.M. Mebed, A.M. Abd-Elnaiem, A.H. Alshammari, T.A. Taha, M. Rashad, D. Hamad, Controlling the structural properties and optical bandgap of PbO-Al2O3 nanocomposites for enhanced photodegradation of methylene blue. Catalysts 12, 142 (2022)
A.M. Abd-Elnaiem, R.F. AbdEl-Baki, F. Alsaaq, S. Orzechowska, D. Hamad, Composite nanoarchitectonics of graphene oxide for better understanding on structural effects on photocatalytic performance for methylene blue dye. J. Inorg. Organomet. Polym. Mater. (2021). https://doi.org/10.1007/s10904-021-02146-3
A.M. Abd-Elnaiem, M.A. Abdel-Rahim, A.Y. Abdel-Latief, A.A.-R. Mohamed, K. Mojsilović, W.J. Stępniowski, Fabrication, characterization and photocatalytic activity of copper oxide nanowires formed by anodization of copper foams. Materials 14, 5030 (2021)
A. Çiftlik, T. Günay Semerci, O. Şahin, F. Semerci, Two-dimensional metal–organic framework nanostructures based on 4,4′-sulfonyldibenzoate for photocatalytic degradation of organic dyes. Crystal Growth & Design 21, 3364–3374 (2021)
S.M.A.G. Ulson de Souza, E. Forgiarini, A.A. Ulson de Souza, Toxicity of textile dyes and their degradation by the enzyme horseradish peroxidase (HRP). J. Hazard. Mater. 147, 1073–1078 (2007)
E. Routoula, S.V. Patwardhan, Degradation of anthraquinone dyes from effluents: a review focusing on enzymatic dye degradation with industrial potential. Environ. Sci. Technol. 54, 647–664 (2020)
W.-Y. Cai, Q. Xu, X.-N. Zhao, J.-J. Zhu, H.-Y. Chen, Porous gold-nanoparticle−CaCO3 hybrid material: preparation, characterization, and application for horseradish Peroxidase assembly and direct electrochemistry. Chem. Mater. 18, 279–284 (2006)
G.-Y. Kim, K.-B. Lee, S.-H. Cho, J. Shim, S.-H. Moon, Electroenzymatic degradation of azo dye using an immobilized peroxidase enzyme. J. Hazard. Mater. 126, 183–188 (2005)
F.-Y. Jeng, S.-C. Lin, Characterization and application of PEGylated horseradish peroxidase for the synthesis of poly (2-naphthol). Process Biochem. 41, 1566–1573 (2006)
M. Negahdary, A. Asadi, S. Mehrtashfar, M. Imandar, H. Akbari-Dastjerdi, F. Salahi, A. Jamaleddini, M. Ajdary, A biosensor for determination of H2O2 by use of HRP enzyme and modified CPE with ZnO NPs. Int. J. Electrochem. Sci. 7, 5185–5194 (2012)
J. Cheng, S.M. Yu, P. Zuo, Horseradish peroxidase immobilized on aluminum-pillared interlayered clay for the catalytic oxidation of phenolic wastewater. Water Res. 40, 283–290 (2006)
B. Krajewska, Application of chitin-and chitosan-based materials for enzyme immobilizations: a review. Enzyme Microb. Technol. 35, 126–139 (2004)
Q. Chang, H. Tang, Immobilization of horseradish peroxidase on NH2-modified magnetic Fe3O4/SiO2 particles and its application in removal of 2,4-Dichlorophenol. Molecules 19, 15768–15782 (2014)
H.-L. Jiang, Q. Xu, Porous metal–organic frameworks as platforms for functional applications. Chem. Commun. 47, 3351–3370 (2011)
H. Zhang, J. Nai, L. Yu, X.W.D. Lou, Metal-organic-framework-based materials as platforms for renewable energy and environmental applications. Joule 1, 77–107 (2017)
H. Furukawa, K.E. Cordova, M. O’Keeffe, O.M. Yaghi, The chemistry and applications of metal-organic frameworks. Science 341, 1230444 (2013)
C. Wang, D. Liu, W. Lin, Metal–organic frameworks as a tunable platform for designing functional molecular materials. J. Am. Chem. Soc. 135, 13222–13234 (2013)
X. Lian, Y. Fang, E. Joseph, Q. Wang, J. Li, S. Banerjee, C. Lollar, X. Wang, H.-C. Zhou, Enzyme–MOF (metal–organic framework) composites. Chem. Soc. Rev. 46, 3386–3401 (2017)
J. Mehta, N. Bhardwaj, S.K. Bhardwaj, K.-H. Kim, A. Deep, Recent advances in enzyme immobilization techniques: metal-organic frameworks as novel substrates. Coord. Chem. Rev. 322, 30–40 (2016)
N.M. Mahmoodi, J. Abdi, Metal-organic framework as a platform of the enzyme to prepare novel environmentally friendly nanobiocatalyst for degrading pollutant in water. J. Ind. Eng. Chem. 80, 606–613 (2019)
M. Bilal, M. Adeel, T. Rasheed, H.M.N. Iqbal, Multifunctional metal–organic frameworks-based biocatalytic platforms: recent developments and future prospects. J. Market. Res. 8, 2359–2371 (2019)
J.H. Cavka, S. Jakobsen, U. Olsbye, N. Guillou, C. Lamberti, S. Bordiga, K.P. Lillerud, A new zirconium inorganic building brick forming metal organic frameworks with exceptional stability. J. Am. Chem. Soc. 130, 13850–13851 (2008)
C. Gomes Silva, I. Luz, F.X. Llabres I Xamena, A. Corma, H. García, Water stable Zr-benzenedicarboxylate metal–organic frameworks as photocatalysts for hydrogen generation. Chemistry 16, 11133–11138 (2010)
Q. Chen, Q. He, M. Lv, Y. Xu, H. Yang, X. Liu, F. Wei, Selective adsorption of cationic dyes by UiO-66-NH2. Appl. Surf. Sci. 327, 77–85 (2015)
G.W. Peterson, J.B. DeCoste, F. Fatollahi-Fard, D.K. Britt, Engineering UiO-66-NH2 for toxic gas removal. Ind. Eng. Chem. Res. 53, 701–707 (2014)
J. Li, F. Wu, L. Lin, Y. Guo, H. Liu, X. Zhang, Flow fabrication of a highly efficient Pd/UiO-66-NH2 film capillary microreactor for 4-nitrophenol reduction. Chem. Eng. J. 333, 146–152 (2018)
S.-L. Cao, D.-M. Yue, X.-H. Li, T.J. Smith, N. Li, M.-H. Zong, H. Wu, Y.-Z. Ma, W.-Y. Lou, Novel nano-/micro-biocatalyst: soybean epoxide hydrolase immobilized on UiO-66-NH2 MOF for efficient biosynthesis of enantiopure (R)-1,2-octanediol in deep eutectic solvents. ACS Sustain. Chem. Eng. 4, 3586–3595 (2016)
X. Chang et al., Tandem biocatalysis by CotA-TJ102@ UIO-66-NH 2 and Novozym 435 for highly selective transformation of HMF into FDCA. Trans. Tianjin Univ. 25, 488–496 (2019)
Y. Li, J. Liu, K. Zhang, L. Lei, Z. Lei, UiO-66-NH2@ PMAA: a hybrid polymer–MOFs architecture for pectinase immobilization. Ind. Eng. Chem. Res. 57, 559–567 (2018)
X. Lian, Y.-P. Chen, T.-F. Liu, H.-C. Zhou, Coupling two enzymes into a tandem nanoreactor utilizing a hierarchically structured MOF. Chem. Sci. 7, 6969–6973 (2016)
E. Gkaniatsou, C. Sicard, R. Ricoux, L. Benahmed, F. Bourdreux, Q. Zhang, C. Serre, J.-P. Mahy, N. Steunou, Enzyme encapsulation in mesoporous metal–organic frameworks for selective biodegradation of harmful dye molecules. Angew. Chem. Int. Ed. 57, 16141–16146 (2018)
X. Liu, W. Qi, Y. Wang, R. Su, Z. He, A facile strategy for enzyme immobilization with highly stable hierarchically porous metal–organic frameworks. Nanoscale 9, 17561–17570 (2017)
M. Kandiah et al., Synthesis and stability of tagged UiO-66 Zr-MOFs. Chem. Mater. 22, 6632–6640 (2010)
W. Chen, J. Chen, Y.-B. Feng, L. Hong, Q.-Y. Chen, L.-F. Wu, X.-H. Lin, X.-H. Xia, Peroxidase-like activity of water-soluble cupric oxide nanoparticles and its analytical application for detection of hydrogen peroxide and glucose. Analyst 137, 1706–1712 (2012)
C.C. Allain, L.S. Poon, C.S.G. Chan, W. Richmond, P.C. Fu, Enzymatic determination of total serum cholesterol. Clin. Chem. 20, 470–475 (1974)
S. Asad, K. Khajeh, N. Ghaemi, Investigating the structural and functional effects of mutating asn glycosylation sites of horseradish peroxidase to asp. Appl. Biochem. Biotechnol. 164, 454–463 (2011)
J. Shanahan, D.S. Kissel, E. Sullivan, PANI@UiO-66 and PANI@UiO-66-NH2 polymer-MOF hybrid composites as tunable semiconducting materials. ACS Omega 5, 6395–6404 (2020)
L. Valenzano, B. Civalleri, S. Chavan, S. Bordiga, M.H. Nilsen, S. Jakobsen, K.P. Lillerud, C. Lamberti, Disclosing the complex structure of uio-66 metal organic framework: a synergic combination of experiment and theory. Chem. Mater. 23, 1700–1718 (2011)
C.F. Macrae, P.R. Edgington, P. McCabe, E. Pidcock, G.P. Shields, R. Taylor, M. Towler, J. Streek, Mercury: visualization and analysis of crystal structures. J. Appl. Crystallogr. 39, 453–457 (2006)
R. Shashanka, H. Esgin, V.M. Yilmaz, Y. Caglar, Fabrication and characterization of green synthesized ZnO nanoparticle based dye-sensitized solar cells. J. Sci. 5, 185–191 (2020)
H. Zeng, Z. Yu, L. Shao, X. Li, M. Zhu, Y. Liu, X. Feng, X. Zhu, Ag2CO3@UiO-66-NH2 embedding graphene oxide sheets photocatalytic membrane for enhancing the removal performance of Cr(VI) and dyes based on filtration. Desalination 491, 114558 (2020)
M. Li, J. Chen, W. Wu, Y. Fang, S. Dong, Oxidase-like MOF-818 nanozyme with high specificity for catalysis of catechol oxidation. J. Am. Chem. Soc. 142, 15569–15574 (2020)
H.-Q. Zheng, C.-Y. Liu, X.-Y. Zeng, J. Chen, J. Lü, R.-G. Lin, R. Cao, Z.-J. Lin, J.-W. Su, MOF-808: A Metal-Organic framework with intrinsic peroxidase-like catalytic activity at neutral pH for colorimetric biosensing. Inorg. Chem. 57, 9096–9104 (2018)
M.J. Uddin, R.E. Ampiaw, W. Lee, Adsorptive removal of dyes from wastewater using a metal-organic framework: a review. Chemosphere 284, 131314 (2021)
T. Alp Arici, O.Z. Yeşilel, M. Arici, A water-stable 2D+2D→3D polycatenated coordination polymer for selective adsorption of methylene blue and detection of Fe3+ ion from aqueous solution. J. Taiwan Inst. Chem. Eng. 114, 300–310 (2020)
T. Deveci, A. Unyayar, M.A. Mazmanci, Production of Remazol Brilliant Blue R decolourising oxygenase from the culture filtrate of Funalia trogii ATCC 200800. J. Mol. Catal. B Enzym. 30, 25–32 (2004)
S.V. Mohan, K.K. Prasad, N.C. Rao, P.N. Sarma, Acid azo dye degradation by free and immobilized horseradish peroxidase (HRP) catalyzed process. Chemosphere 58, 1097–1105 (2005)
W. Liang, P. Wied, F. Carraro, C.J. Sumby, B. Nidetzky, C.-K. Tsung, P. Falcaro, C.J. Doonan, Metal–organic framework-based enzyme biocomposites. Chem. Rev. 121, 1077–1129 (2021)
Y. Du et al., Metal-organic frameworks with different dimensionalities: an ideal host platform for enzyme@MOF composites. Coord. Chem. Rev. 454, 214327 (2022)
S.S. Nadar, L. Vaidya, V.K. Rathod, Enzyme embedded metal organic framework (enzyme–MOF): de novo approaches for immobilization. Int. J. Biol. Macromol. 149, 861–876 (2020)
S. Tadepalli, J. Yim, S. Cao, Z. Wang, R.R. Naik, S. Singamaneni, Metal–organic framework encapsulation for the preservation and photothermal enhancement of enzyme activity. Small 14, 1702382 (2018)
Acknowledgements
The authors gratefully acknowledge the financial support from Kırklareli University through Project No. KLUBAP-140.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kurtuldu, A., Eşgin, H., Yetim, N.K. et al. Immobilization Horseradish Peroxidase onto UiO-66-NH2 for Biodegradation of Organic Dyes. J Inorg Organomet Polym 32, 2901–2909 (2022). https://doi.org/10.1007/s10904-022-02310-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10904-022-02310-3