Abstract
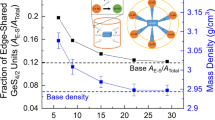
The melt-quenching method has been used to fabricate a PbO2–B2O3–CeO2 glass system. The XRD diffractometer procedure was used to check the status of these samples. It can be concluded, from the X-ray diffraction curves, that the tested samples have high levels of glassiness. As the CeO2 concentration increased most of the [BO4] are converted into [BO3] structural units with the formation of non-bridging oxygen ions in the borate matrix. It can be cross verified with the decrease of the N4 fraction from 0.654 to 0.239. This decrease may be attributed to the formation of [CeO7] structural units that needs more oxygen atoms. The ultrasonic velocities of the prepared glasses are decreased with the increase of CeO2 content. Different types of elastic modules were evaluated (experimental and theoretical) for the prepared glasses are increases with the increase of CeO2 content. Glass transition temperature (Tg), crystallization temperature (Tc), the peak of crystallization temperature (Tp) and thermal stability values decreases with the increase of CeO2 content. The refractive index of these samples is increasing with the increase in the reflection and the density.























Similar content being viewed by others
References
K.S. Shaaban, Y.B. Saddeek, Effect of MoO3 content on structural, thermal, mechanical and optical properties of (B2O3-SiO2-Bi2O3-Na2O-Fe2O3) glass system. Silicon 9(5), 785–793 (2017)
N. Singh, K.J. Singh, K. Singh, H. Singh, Comparative study of lead borate and bismuth lead borate glass systems as gamma-radiation shielding materials. Nucl. Instrum. Methods Phys. Res. Sect. B 225, 305–309 (2004)
K.S. Shaaban, A.M. Ali, Y.B. Saddeek et al., Synthesis, mechanical and optical features of Dy2O3 doped lead alkali borosilicate glasses. Silicon 11, 1853–1861 (2019)
S. Kaur, K.J. Singh, Comparative study of lead borate and lead silicate glass systems doped with aluminum oxide as gamma-ray shielding materials. Int. J. Innov. Technol. Explor. Eng. 172, 2278–3075 (2013)
K.S. Shaaban, E.A.A. Wahab, E.R. Shaaban et al., Electronic polarizability, optical basicity, thermal, mechanical and optical investigations of (65B2O3–30Li2O–5Al2O3) glasses doped with titanate. J. Electron. Mater. 49, 2040–2049 (2020). https://doi.org/10.1007/s11664-019-07889-x
W.M. Abd-Allah, H.A. Saudi, K.S. Shaaban et al., Investigation of structural and radiation shielding properties of 40B2O3–30PbO–(30–x) BaO-x ZnO glass system. Appl. Phys. A 125, 275 (2019)
R.M. El-Sharkawy, K.S. Shaaban, R. Elsaman, E.A. Allam, A. El-Taher, M.E. Mahmoud, Investigation of mechanical and radiation shielding characteristics of novel glass systems with the composition xNiO-20ZnO-60B2O3-(20–x) CdO based on nano metal oxides. J. Non-Cryst. Solids 528, 119754 (2020)
E.A. Abdel Wahab, K.S. Shaaban, R. Elsaman, E.S. Yousef, Radiation shielding, and physical properties of lead borate glass doped ZrO2 nanoparticles. Appl. Phys. A 125(12), 869 (2019)
A.M. Fayad, K.S. Shaaban, W.M. Abd-Allah et al., Structural and optical study of CoO doping in borophosphate host glass and effect of gamma irradiation. J. Inorg. Organomet. Polym. (2020). https://doi.org/10.1007/s10904-020-01641-3
H.A. Saudi, W.M. Abd-Allah, K.S. Shaaban, Investigation of gamma and neutron shielding parameters for borosilicate glasses doped europium oxide for the immobilization of radioactive waste. J. Mater. Sci. 31, 6963–6976 (2020)
M.A. Azooz, Y.B. Saddeek, K.A. Aly et al., Optical, infrared spectral and mechanical investigations of CeO2-doped borosilicate glasses containing Bi2O3 and TeO2. J. Inorg. Organomet. Polym. 29, 1680–1687 (2019)
M.A. Marzouk, I.S. Ali, H.A. ElBatal, Optical, FT infrared and photoluminescence spectra of CeO2—doped Na2O–ZnO–B2O3 host glass and effects of gamma irradiation. J. Non-Cryst. Solids 485, 14–23 (2018)
S. Gómez-Salces, J.A. Barreda-Argüeso, R. Valiente, F. Rodríguez, Solarization-induced redox reactions in doubly Ce3+ /Mn2+—highly doped transmission glasses studied by optical absorption and photoluminescence. Sol. Energy Mater. Sol. Cells 157, 42–47 (2016)
Q. Chen, Y. Qiao, H. Wang, Q. Chen, Spectra and magneto optical behavior of CeO2 doped heavy metal diamagnetic glass. J. Non-Cryst. Solids 470, 70–77 (2017)
G.P. Singh, P. Kaur, S. Kaur, D.P. Singh, Conversion of covalent to ionic character of V2O5–CeO2–PbO–B2O3 glasses for solid state ionic devices. Phys. B 407, 4269–4273 (2012)
K.S. Shaaban, E.S. Yousef, E.A. Abdel Wahab et al., Investigation of crystallization and mechanical characteristics of glass and glass-ceramic with the compositions xFe2O3-35SiO2-35B2O3-10Al2O3-(20–x) Na2O. J. Mater. Eng. Perform. (2020). https://doi.org/10.1007/s11665-020-04969-6
H.H. Somaily, K.S. Shaaban, S.A. Makhlouf et al., Comparative studies on polarizability, optical basicity and optical properties of lead borosilicate modified with titania. J. Inorg. Organomet. Polym. (2020). https://doi.org/10.1007/s10904-020-01650-2
K.S. Shaaban, S.M. Abo-Naf, M.E.M. Hassouna, Physical and structural properties of lithium borate glasses containing MoO3. Silicon 11, 2421–2428 (2019)
K.S. Shaaban, S.M. Abo-naf, A.M. Abd Elnaeim, M.E.M. Hassouna, Studying effect of MoO3 on elastic and crystallization behavior of lithium diborate glasses. Appl. Phys. A 123(6), 457–466 (2017)
A.M. Efimov, Vibrational spectra, related properties, and structure of inorganic glasses. J. Non-Crystalline Solids 253(1–3), 95–118 (1999)
J. Wong, C.A. Angell, Glass Structure by Spectroscopy (Marcel Dekker, New York, 1976)
G. El-Damrawi, K. El-Egili, Characterization of novel CeO2–B2O3 glasses, structure, and properties. Phys. B 299(1–2), 180–186 (2001)
E.A.A. Wahab, K.S. Shaaban, Effects of SnO2 on spectroscopic properties of borosilicate glasses before and after plasma treatment and its mechanical properties. Mater. Res. Express 5(2), 025207 (2018)
K.S. Shaaban, E.S. Yousef, S.A. Mahmoud et al., Mechanical, structural and crystallization properties in titanate doped phosphate glasses. J Inorg Organomet Polym (2020). https://doi.org/10.1007/s10904-020-01574-x
K.S. Shaaban, M.S.I. Koubisy, H.Y. Zahran et al., Spectroscopic Properties, Electronic Polarizability, and Optical Basicity of Titanium-Cadmium Tellurite Glasses Doped with Different Amounts of Lanthanum. J Inorg Organomet Polym (2020). https://doi.org/10.1007/s10904-020-01640-4
E.I. Kamitsos, A.P. Patsis, M.A. Karakassides, G.D. Chryssikos, Infrared reflectance spectra of lithium borate glasses. J. Non-Cryst. Solids 126(1–2), 52–67 (1990)
C. Julien, M. Massot, W. Balkanski, A. Krol, W. Nazarewicz, Infrared studies of the structure of borate glasses. Mater. Sci. Eng., B 3(3), 307–312 (1989)
E.I. Kamitsos, Infrared studies of borate glasses. Phys. Chem. Glasses Eur. J. Glass Sci. Technol. Part B 44(2), 79–87 (2003)
A.A. El-Maaref, E.A.A. Wahab, K.S. Shaaban, M. Abdelawwad, M.S.I. Koubisy, J. Börcsök, E.S. Yousef, Visible and mid-infrared spectral emissions and radiative rates calculations of Tm3+ doped BBLC glass. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 242, 118774 (2020)
E.A. AbdelWahab, K.S. Shaaban, E.S. Yousef, Enhancement of optical and mechanical properties of sodium silicate glasses using zirconia. Opt. Quant. Electron. 52, 458 (2020). https://doi.org/10.1007/s11082-020-02575-3
K.S. Shaaban, H.Y. Zahran, I.S. Yahia et al., Mechanical and radiation-shielding properties of B2O3–P2O5–Li2O–MoO3 glasses. Appl. Phys. A 126, 804 (2020). https://doi.org/10.1007/s00339-020-03982-9
K.S. Shaaban, E.A.A. Wahab, E.R. Shaaban et al., Electronic polarizability, optical basicity, and mechanical properties of aluminum lead phosphate glasses. Opt. Quant. Electron. 52, 125 (2020)
A. Gaddam, H.R. Fernandes, B. Doumert, L. Montagne, J.M.F. Ferreira, Structure and thermal relaxation of network units and crystallization of lithium silicate-based glasses doped with oxides of Al and B. Phys. Chem. Chem. Phys 19(38), 26034–26046 (2017). https://doi.org/10.1039/c7cp01690e
S. Ibrahim, M.M. Gomaa, H. Darwish, Influence of Fe2O3 on the physical, structural and electrical properties of sodium lead borate glasses. J. Adv. Ceramics 3(2), 155–164 (2014). https://doi.org/10.1007/s40145-014-0107-z
K.S. Shaaban, E.S. Yousef, Optical properties of Bi2O3 doped boro tellurite glasses and glass ceramics. Optik Int. J. Light Electron. Opt. 203, 163976 (2020)
A.F.A. El-Rehim, K.S. Shaaban, H.Y. Zahran et al., Structural and mechanical properties of lithium bismuth borate glasses containing molybdenum (LBBM) together with their glass-ceramics. J Inorg Organomet Polym (2020). https://doi.org/10.1007/s10904-020-01708-1
D.P. Singh, G. Pal Singh, Conversion of covalent to ionic behavior of Fe2O3–CeO2–PbO–B2O3 glasses for ionic and photonic application. J. Alloys Compd. 546, 224–228 (2013). https://doi.org/10.1016/j.jallcom.2012.08.105
J.A. Duffy, Ionic−covalent character of metal and nonmetal oxides. J. Phys. Chem. A 110(49), 13245–13248 (2006). https://doi.org/10.1021/jp063846j
V. Dimitrov, S. Sakka, Linear and nonlinear optical properties of simple oxides II. J. Appl. Phys. 79(3), 1741–1745 (1996). https://doi.org/10.1063/1.360963
T.R. Tasheva, V.V. Dimitrov, Electronic polarizability, optical basicity and chemical bonding of zinc oxide-barium oxide-vanadium oxide glasses. Bulg. Chem. Commun 49(Special Issue F), 76–83 (2017)
M. Gaafar, S. Marzouk, Mechanical and structural studies on sodium borosilicate glasses doped with Er2O3 using ultrasonic velocity and FTIR spectroscopy. Phys. B 338, 294–302 (2007)
A. Makishima, J.D. Mackenzie, Direct calculation of young’s moidulus of glass. J. Non-Cryst. Solids 12(1), 35–45 (1973)
A. Makishima, J.D. Mackenzie, Calculation of bulk modulus, shear modulus and Poisson’s ratio of glass. J. Non-Cryst. Solids 17(2), 147–157 (1975)
K.H.S. Shaaban, Y. Saddeek, K. Aly, Physical properties of pseudo quaternary Na2B4O7–SiO2–MoO3–Dy2O3 glasses. Ceram. Int. 44, 3862–3867 (2018)
U. Veit, C. Rüssel, Elastic properties of quaternary glasses in the MgO–CaO–Al2O3–SiO2 system: modelling versus measurement. J. Mater. Sci. 52, 8159–8175 (2017)
T.A. Taha, A.S. Abouhaswa, Preparation and optical properties of borate glass doped with MnO2. J. Mater. Sci. 29(10), 8100 (2018)
K. Shaaban, E.A. Abdel Wahab, A.A. El-Maaref et al., Judd-Ofelt analysis and physical properties of erbium modified cadmium lithium gadolinium silicate glasses. J Mater Sci 31, 4986–4996 (2020)
B.C. Yadav, R.C. Yadav, S. Singh, P.K. Dwivedi, H. Ryu, S. Kang, Nanostructured cobalt oxide and cobalt titanate thin films as optical humidity sensor: a new approach. Opt. Laser Technol. 49, 68–74 (2013)
A.A. El-Maaref, S. Badr, K.S. Shaaban, E.A.A. Wahab, M.M. El Okr, Optical properties and radiative rates of Nd3+ doped zinc-sodium phosphate glasses. J. Rare Earths 37, 253–259 (2019)
F. Urbach, The long-wavelength edge of photographic sensitivity and of the electronic absorption of solids. Phys. Rev. 92(5), 1324–1324 (1953)
Z.A. Said Mahraz, M.R. Sahar, S.K. Ghoshal, M. Reza Dousti, Concentration dependent luminescence quenching of Er3+-doped zinc boro-tellurite glass. J. Lumin. 144, 139–145 (2013)
T.S. Moss, Relations between the refractive index and energy gap of semiconductors. Physica Status Solidi (b) 131(2), 415–427 (1985). https://doi.org/10.1002/pssb.2221310202
N.M. Ravindra, Energy gap-refractive index relation—some observations. Infrared Phys. 21(5), 283–285 (1981). https://doi.org/10.1016/0020-0891(81)90033-6
V.P. Gupta, N.M. Ravindra, Comments on the moss formula. Physica Status Solidi (b) 100(2), 715–719 (1980). https://doi.org/10.1002/pssb.2221000240
M. Anani, C. Mathieu, S. Lebid, Y. Amar, Z. Chama, H. Abid, Model for calculating the refractive index of a III-V semiconductor. Comput. Mater. Sci 41, 570–757 (2008)
V. Kumar, J.K. Singh, Model for calculating the refractive index of different materials. Ind. J. Pure Appl. Phys. 48, 571–574 (2010)
P. Hervé, L.K.J. Vandamme, General relation between refractive index and energy gap in semiconductors. Infrared Phys. Technol. 35(4), 609–615 (1994). https://doi.org/10.1016/1350-4495(94)90026-4
Acknowledgment
The authors extend their appreciation to the Deanship of Scientific Research at King Khalid University for funding this work through research groups program under Grant Number R.G.P. 2/93/41.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Declaration of interes
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
El-Rehim, A.F.A., Ali, A.M., Zahran, H.Y. et al. Spectroscopic, Structural, Thermal, and Mechanical Properties of B2O3-CeO2-PbO2 Glasses. J Inorg Organomet Polym 31, 1774–1786 (2021). https://doi.org/10.1007/s10904-020-01799-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10904-020-01799-w