Abstract
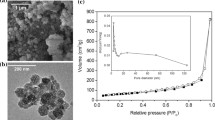
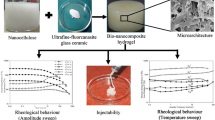
Injectable biomaterials have gained significant attention in recent years since they can be delivered to the defect sites through minimally-invasive approaches. In this work, thermo-responsive injectable hydrogels containing borate-based bioactive glass particles and carbon-based nanopowders were designed for bone tissue engineering applications. For this purpose, mixtures of Pluronic F127 block-copolymer and 13-93B3 bioactive glass particles with different sizes (2.3 µm, 14 µm, 150 µm) were prepared in aqueous medium and their in situ gelation were investigated through rheological measurements as a function of temperature. Effects of graphene nanopowders and multi-walled carbon nanotubes on the flow behavior of the designed hydrogel system were also investigated. Results revealed that viscosity of the prepared hydrogel system was strongly dependent on the temperature and the bioactive glass particle size. Inclusion of graphene and multi-walled carbon nanotubes in this system caused a further increase in viscosity. All of the hydrogel compositions designed in the study showed shear thinning flow behavior which is a crucial parameter for injectability. Drug release studies showed that the addition of bioactive glass and carbon-based nanoparticles improved the drug release behavior of the prepared hydrogels.










Similar content being viewed by others
References
H. Ding, C.-J. Zhao, X. Cui, Y.-F. Gu, W.-T. Jia, M.N. Rahaman, Y. Wang, W.-H. Huang, C.-Q. Zhang, Novel injectable borate bioactive glass cement as an antibiotic delivery vehicle for treating osteomyelitis. PLoS ONE 9(1), e85472 (2014)
X. Liu, X. Wang, Z. Chen, F.Z. Cui, H.Y. Liu, K. Mao, Y. Wang, Injectable bone cement based on mineralized collagen. J. Biomed. Mater. Res. 94, 72–79 (2010)
T. Hui, X. Yongqing, Z. Tiane, L. Gang, Y. Yongqang, J. Muyao, L. Jun, D. Jing, Treatment of osteomyelitis by liposomal gentamicin-impregnated calcium sulfate. Arch. Orthop. Trauma Surg. 129, 1301–1308 (2009)
W. Jia, S.H. Luo, C.Q. Zhang, J.Q. Wang, In vitro and in vivo efficacies of teicoplanin-loaded calcium sulfate for treatment of chronic methicillin-resistant Staphylococcus aureus osteomyelitis. Antimicrob. Agents Chemother. 54, 170–176 (2010)
M.A. Rauschmann, T.A. Wichelhaus, V. Stirnal, E. Dingeldein, L. Zichner, Nanocrystalline hydroxyapatite and calcium sulphate as biodegradable composite carrier material for local delivery of antibiotics in bone infections. Biomaterials 26, 2677–2684 (2005)
J.A. Killion, S. Kehoe, L.M. Geever, D.M. Devine, E. Sheehan, D. Boyd, C.L. Higginbotham, Hydrogel/bioactive glass composites for bone regeneration applications: synthesis and characterisation. Mater. Sci. Eng. C 33, 4203–4212 (2013)
N.L. Elstad, K.D. Fowers, OncoGel (ReGel/paclitaxel)—clinical applications for a novel paclitaxel delivery system. Adv. Drug Deliv. Rev. 61, 785–794 (2009)
S. Nie, W.L.W. Hsiao, W. Pan, Z. Yang, Thermoreversible Pluronic® F127-based hydrogel containing liposomes for the controlled delivery of paclitaxel: in vitro drug release, cell cytotoxicity, and uptake studies. Int. J. Nanomed. 6, 151–166 (2011)
J. Liaw, Y. Lin, Evaluation of poly(ethylene oxide)–poly(propylene oxide)–poly(ethylene oxide) (PEO–PPO–PEO) gels as a release vehicle for percutaneous fentanyl. J. Control. Release 68, 273–282 (2000)
P.J. Kondiah, Y.E. Choonara, P.P.D. Kondiah, T. Marimuthu, P. Kumar, L.C. du Toit, V. Pillay, A review of injectable polymeric hydrogel systems for application in bone tissue engineering. Molecules 21, 1580 (2016). https://doi.org/10.3390/molecules21111580
M. Bercea, S. Morariu, L.E. Nita, R.N. Darie, Investigation of poly(vinyl alcohol)/pluronic F127 physical gels. Polym.-Plast. Technol. Eng. 53, 1354–1361 (2014)
L.C.P. Trong, M. Djabourov, A. Ponton, Mechanisms of micellization and rheology of PEO-PPO-PEO triblock copolymers with different architectures. J. Colloid Interfaces. Sci. 328, 278–287 (2008)
K. Derakhshandeh, M. Fashi, S. Seifoleslami, Thermosensitive Pluronic® hydrogel: prolonged injectable formulation for drug abuse. Drug Des. Dev. Ther. 4, 255–262 (2010)
J.J. Escobar-Chávez, M. López-Cervantes, A. Naïk, Y.N. Kalia, D. Quintanar-Guerrero, A. Ganem-Quintanar, Applications of thermoreversible Pluronic F-127 gels in pharmaceutical formulations. J. Pharm. Pharmaceut. Sci. 9, 339–358 (2006)
G. Cirillo, S. Hampel, U.G. Spizzirri, O.I. Parisi, N. Picci, F. Iemma, Carbon nanotubes hybrid hydrogels in drug delivery: a perspective review. Biomed. Res. Int. 2014, 825017 (2014)
Y.-S. Jung, W. Park, H. Parka, D.-K. Leeb, K. Na, Thermo-sensitive injectable hydrogel based on the physical mixing of hyaluronicacid and Pluronic F-127 for sustained NSAID delivery. Carbohyd. Polym. 156, 403–408 (2017)
J. Clapper, J. Skeie, R. Mullins, C.A. Guymon, Development and characterization of photopolymerizable biodegradable materials from PEG–PLA–PEG block macromonomers. Polymer 48, 6554–6564 (2007)
E. Lippens, G. Vertenten, J. Gironès, H. Declercq, J. Saunders, J. Luyten, L. Duchateau, E. Schacht, L. Vlaminck, F. Gasthuys, M. Cornelissen, Evaluation of bone regeneration with an injectable, in situ polymerizable Pluronic® F127 hydrogel derivative combined with autologous mesenchymal stem cells in a goat tibia defect model. Tissue Eng. Part A 16(2), 617–627 (2010)
N.S. Satarkar, D. Johnson, B. Marrs, R. Andrews, C. Poh, B. Gharaibeh, K. Saito, K.W. Anderson, J.Z. Hilt, Hydrogel-MWCNT nanocomposites: synthesis, characterization, and heating with radiofrequency fields. J. Appl. Polym. Sci. 117(3), 1813–1819 (2010)
P. Schexnailder, G. Schmidt, Nanocomposite polymer hydrogels. Colloid Polym. Sci. 287(1), 1–11 (2009)
H. Hu, J. Yu, Y. Li, J. Zhao, H. Dong, Engineering of a novel pluronic F127/graphene nanohybrid for pH responsive drug delivery. J. Biomed. Mater. Res. Part A 100A, 141–148 (2012)
J.S. Muñoz, U. Kettenberger, P. Procter, D.P. Pioletti, Non-setting, injectable biomaterials containing particulate hydroxyapatite can increase primary stability of bone screws in cancellous bone. Clin. Biomech. 59, 174–180 (2018)
C. Pontremoli, M. Boffito, S. Fiorilli, R. Laurano, A. Torchio, A. Bari, C. Tonda-Turo, G. Ciardelli, C. Vitale-Brovarone, Hybrid injectable platforms for the in situ delivery of therapeutic ions from mesoporous glasses. Chem. Eng. J. 340, 103–113 (2018)
L. Bi, S. Jung, D. Day, K. Neidig, V. Dusevich, D. Eick, L. Bonewald, Evaluation of bone regeneration, angiogenesis, and hydroxyapatite conversion in critical sized rat calvarial defects implanted with bioactive glass scaffolds. J. Biomed. Mater. Res. 100, 3267–3275 (2012)
Q.Z. Chen, I.D. Thompson, A.R. Boccaccini, 45S5 Bioglass-derived glass-ceramic scaffolds for bone tissue engineering. Biomaterials 27, 2414–2425 (2006)
L.L. Hench, R.J. Splinter, W.C. Allen, T.K. Greenlee, Bonding mechanisms at the interface of ceramic prosthetic materials. J. Biomed. Mater. Res. 5, 117–141 (1971)
E. Fiume, J. Barberi, E. Verné, F. Baino, Bioactive glasses: from parent 45S5 composition to scaffold-assisted tissue-healing therapies. J. Funct. Biomater. 9, 24 (2018). https://doi.org/10.3390/jfb9010024
M. Brink, The influence of alkali and alkaline earths on the working range for bioactive glasses. J. Biomed. Mater. Res. 36(1), 109–117 (1997)
F. Baino, S. Hamzehlou, S. Kargozar, Bioactive glasses: where are we and where are we going? J. Funct. Biomater. 9, 25 (2018)
W. Liang, M.N. Rahaman, D.E. Day, N.W. Marion, G.C. Riley, J.J. Mao, Bioactive borate glass scaffold for bone tissue engineering. J. Non-Cryst. Solids 354, 1690–1696 (2008)
H. Fu, Q. Fu, N. Zhou, W. Huang, M.N. Rahaman, D. Wang, X. Liu, In vitro evaluation of borate-based bioactive glass scaffolds prepared by a polymer foam replication method. Mater. Sci. Eng. C 29, 2275–2281 (2009)
M.N. Rahaman, D.E. Day, B.S. Bal, Q. Fu, S.B. Jung, L.F. Bonewalde, A.P. Tomsia, Bioactive glass in tissue engineering. Acta Biomater. 7, 2355–2373 (2011)
S. Naseri, W.C. Lepry, S.N. Nazhat, Bioactive glasses in wound healing: hope or hype? J. Mater. Chem. B 5, 6167–6174 (2017)
X. Cui, Y. Zhang, H. Wang, Y. Gu, L. Li, J. Zhou, S. Zhao, W. Huang, N. Zhou, D. Wang, H. Pan, M.N. Rahaman, An injectable borate bioactive glass cement for bone repair: preparation, bioactivity and setting mechanism. J. Non-Cryst. Solids 432, 150–157 (2016)
G. Jin, K. Li, The electrically conductive scaffold as the skeleton of stem cell niche in regenerative medicine. Mater. Sci. Eng. C 45, 671–681 (2014)
M. Türk, A.M. Deliormanlı, Electrically conductive porous borate-based bioactive glass scaffolds for bone tissue engineering applications. J. Biomater. Appl. 32(1), 28–39 (2017)
B. Li, L. Zhang, Z. Zhang, R. Gao, H. Li, Z. Dong, Q. Wang, Q. Zhou, Y. Wang, Physiologically stable F127-GO supramolecular hydrogel with sustained drug release characteristic for chemotherapy and photothermal therapy. RSC Adv. 8, 1693–1699 (2018)
A. Yao, D. Wang, W. Huang, Q. Fu, M.N. Rahaman, D.E. Day, In vitro bioactive characteristics of borate-based glasses with controllable degradation behavior. J. Am. Ceram. Soc. 90(1), 303–306 (2007)
T. Kokubo, H. Kushitani, S. Sakka, T. Kitsugi, T. Yamamuro, Solutions able to reproduce in vivo surface-structure changes in bioactive glass-ceramic A-W. J. Biomed. Mater. Res. 24, 721–734 (1990)
K. Kurumada, B.H. Robinson, Viscosity studies of Pluronic F127 in aqueous solution. Progr. Colloid Polm. Sci. 123, 12–15 (2004)
M.T. Cidade, D.J. Ramos, J. Santos, H. Carrelo, N. Calero, J.P. Borges, Injectable hydrogels based on Pluronic/water systems filled with alginate microparticles for biomedical applications. Materials 12, 1083 (2019). https://doi.org/10.3390/ma12071083
B.K. Lau, Q. Wang, W. Sun, L. Li, Micellization to gelation of a triblock copolymer in water: thermoreversibility and scaling. J. Polym. Sci. B 42, 2014–2025 (2004)
A. Peigney, C. Laurent, E. Flahaut, R.R. Bacsa, A. Rousset, Specific surface area of carbon nanotubes and bundles of carbon nanotubes. Carbon 39(4), 507–514 (2001)
M. Guvendiren, H.D. Lua, J.A. Burdick, Shear-thinning hydrogels for biomedical applications. Soft Matter 8, 260–272 (2012). https://doi.org/10.1039/C1SM06513K
X. Xu, S.A. Rice, A.R. Dinner, Relation between ordering and shear thinning in colloidal suspensions. Proc. Natl. Acad. Sci. 110(10), 3771–3776 (2013). https://doi.org/10.1073/pnas.1301055110
A. Vázquez-Quesada, R.I. Tanner, M. Ellero, Shear thinning of noncolloidal suspensions. Phys. Rev. Lett. 117, 108001 (2016)
Y. Ryabenkova, A. Pinnock, P.A. Quadros, R.L. Goodchild, G. Möbus, A. Crawford, P.V. Hatton, C.A. Miller, The relationship between particle morphology and rheological properties in injectable nano-hydroxyapatite bone graft substitutes. Mater. Sci. Eng. 75, 1083–1090 (2017)
H.A. Barnes, J.F. Hutton, K. Walters, An Introduction to Rheology, 1st edn. (Elsevier, Amsterdam, 1989)
D.B. Genovese, Shear rheology of hard-sphere, dispersed, and aggregated suspensions, and filler-matrix composites. Adv. Colloid Interface 171–172, 1–16 (2012)
S.K. Kawatra, T.C. Eisele, Rheological effects in grinding circuits. Int. J. Miner. Process. 22, 251–259 (1988)
N. Mangesana, R.S. Chikuku, A.N. Mainza, I. Govender, A.P. van der Westhuizen, M. Narashima, The effect of particle sizes and solids concentration on the rheology of silica sand based suspensions. J. S. Afr. Inst. Min. Metall. 108, 237–243 (2008)
P.F. Luckham, M.A. Ukeje, Effect of particle size distribution on the rheology of dispersed systems. J. Colloid Interfaces Sci. 220, 347–356 (1999)
Z. Zhou, M.J. Solomon, P.J. Scales, D.V. Boger, The yield stress of concentrated flocculated suspensions of size distributed particles. J. Rheol. 43, 651 (1999). https://doi.org/10.1122/1.551029
R.J. Farris, Prediction of the viscosity of multimodal suspensions from unimodal viscosity data. Trans. Soc. Rheol. 12, 281–301 (1968)
C. Ancey, Role of lubricated contacts in concentrated polydisperse suspensions. J. Rheol. 45, 1421–1439 (2001). https://doi.org/10.1122/1.1413504
W. Pabst, E. Gregorova, C. Berthold, Particle shape and suspension rheology of short-fiber systems. J. Eur. Ceram. Soc. 26, 149–160 (2006). https://doi.org/10.1016/j.jeurceramsoc.2004.10.016
B. Klein, S.J. Partridge, J.S. Laskowski, Influence of physicomechanical properties on the rheology and stability of magnetite dense media, Production and Processing of Fine Particles, Canadian Institute of Mining and Metallurgy, ed by A.J. Plumpton, (1988)
Y.V. Shtogun, L.M. Woods, Many-body van der waals interactions between graphitic nanostructures. J. Phys. Chem. Lett. 1, 1356–1362 (2010). https://doi.org/10.1021/jz100309m
V.V. Gobre, A. Tkatchenko, Scaling laws for van der Waals interactions in nanostructured materials. Nat. Commun. 4, 2341 (2013). https://doi.org/10.1038/ncomms3341
R. Atif, F. Inam, Reasons and remedies for the agglomeration of multilayered graphene and carbon nanotubes in polymers. Beilstein J. Nanotechnol. 7, 1174–1196 (2016). https://doi.org/10.3762/bjnano.7.109
O.V. Kharissova, B.I. Kharisov, E.G. de Casas Ortiz, Dispersion of carbon nanotubes in water and non-aqueous solvents. RSC Adv. 3, 24812 (2013)
M. Bohner, G. Baroud, Injectability of calcium phosphate pastes. Biomaterials 26, 1553–1563 (2005)
R. O’Neill, H.O. McCarthy, E. Montufar, M.-P. Ginebra, D.I. Wilson, A. Lennon, N. Dunne, Critical review: injectability of calcium phosphate pastes and cements. Acta Biomater. 50, 1–19 (2017)
M. Farrugia, S.P. Morgan, C. Alexander, M.L. Mather, Ultrasonic monitoring of drug loaded Pluronic F127 micellular hydrogel phase behaviour. Mater. Sci. Eng. C 34, 280–286 (2014)
Q.M. Jiang, Y.F. Fan, C.S. Tao, The experiment of stability of gentamycin sulfate mixture. Med. J. Commun. 16, 291–292 (2002)
S. Chen, L. Ge, A. Mueller, M.A. Carlson, M.J. Teusink, F.D. Shuler, J. Xie, Twisting electrospun nanofiber fine strips into functional sutures for sustained co-delivery of gentamicin and silver. Nanomedicine 13(4), 1435–1445 (2017). https://doi.org/10.1016/j.nano.2017.01.016
M.Y. Krasko, J. Golenser, A. Nyska, M. Nyska, Y.S. Brin, A.J. Domb, Gentamicin extended release from an injectable polymeric implant. J. Controll. Release 117, 90–96 (2007)
J. Liu, L. Cui, D. Losic, Graphene and graphene oxide as new nanocarriers for drug delivery application. Acta Biomater. 9(12), 9243–9257 (2013)
A.M.A. Elhissi, W. Ahmed, I. Ul Hassan, V.R. Dhanak, A. D’Emanuele, Carbon nanotubes in cancer therapy and drug delivery. J. Drug Deliv. 2012, 837327 (2012)
L. Saeednia, L. Yao, K. Cluff, R. Asmatulu, Sustained releasing of methotrexate from injectable and thermosensitive chitosan–carbon nanotube hybrid hydrogels effectively controls tumor cell growth. ACS Omega 4(2), 4040–4048 (2019)
H. Pandey, V. Parashar, R. Parashar, R. Prakash, P.W. Ramteke, A.C. Pandey, Controlled drug release characteristics and enhanced antibacterial effect of graphene nanosheets containing gentamicin sulfate. Nanoscale 3, 4104–4108 (2011)
Z. Xie, X. Cui, C. Zhao, W. Huang, J. Wang, C. Zhang, Gentamicin-loaded borate bioactive glass eradicates osteomyelitis due to Escherichia coli in a rabbit model. Antimicrob. Agents Chemother. 57(7), 3293–3298 (2013). https://doi.org/10.1128/AAC.00284-13
Q. Fu, M.N. Rahaman, H. Fu, X. Liu, Silicate, borosilicate, and borate bioactive glass scaffolds with controllable degradation rate for bone tissue engineering applications. I. Preparation and in vitro degradation. J. Biomed. Mater. Res. A 95A, 164–171 (2010)
A.M. Deliormanlı, Size dependent degradation behavior of borate bioactive glass. Ceram. Int. 39(7), 8087–8095 (2013)
Acknowledgements
The financial support for this research was provided by the Scientific and Technical Research Council of Turkey (TUBITAK), 1001 grant program for scientific and technological research projects; Project No: 114M519.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Deliormanlı, A.M., Türk, M. Flow Behavior and Drug Release Study of Injectable Pluronic F-127 Hydrogels containing Bioactive Glass and Carbon-Based Nanopowders. J Inorg Organomet Polym 30, 1184–1196 (2020). https://doi.org/10.1007/s10904-019-01346-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10904-019-01346-2