Abstract
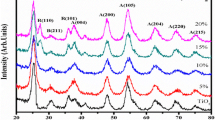
A simple sol–gel method was used to synthesize cobalt doped TiO2 nanoparticles. TiO2 (titania) nanoparticles with Co doping concentration within the range 0, 2, 4, 6 and 8 mol% were prepared. For the purpose of samples characterization under the effect of changing the Co concentration on structural, optical, surface morphology and magnetic properties of the samples X-ray diffraction (XRD), Fourier transform infrared, UV/Vis/NIR diffuse reflectance spectroscopy (DRS), UV–visible absorption, transmission electron microscopy, and vibrating sample magnetometer (VSM) system techniques were employed. The XRD results confirmed the formation of TiO2 (titania) nanoparticles in anatase phase for all the undoped and Co-doped samples. The microstructure studies of the samples confirmed the incorporation of Co ions into the host titania matrix is occurring via substitution for the Ti sites. All the investigated samples exhibited a room temperature ferromagnetic behavior as observed by VSM measurements with non-monotonic dependence of FM characteristic parameters on Co concentration. The samples showed optical energy gap (Eg) values ~ 3.59 eV with an extension of the band gap into the visible light region with Co doping as confirmed by DRS study. The absorption band shows shift from 510 nm to 720 nm as Co concentration in the samples increased.







Similar content being viewed by others
References
S. Maensiri, P. Laokul, J. Klinkaewnarong, A simple synthesis and room-temperature magnetic behavior of Co-doped anatase TiO2 nanoparticles. J. Magn. Magn. Mater. 302, 448–453 (2006)
T.C. Oliveira, E.F. Silva, TiO2 ceramics prepared using Pechini synthesis and laser sintering. Proc. Appl. Ceram. 12, 118–122 (2018)
G.S. Mital, T. Manoj, A review of TiO2 nanoparticles. Chin. Sci. Bull. 56, 1639–1657 (2011)
A. Fujishima, K. Honda, Electrochemical photolysis of water at a semiconductor electrode. Nature 238, 37–38 (1972)
S.A. Mansour, Non-isothermal crystallization kinetics of nano-sized amorphous TiO2 prepared by facile sonochemical hydrolysis route. Ceram. Int. 45, 2893–2898 (2019)
N. Drăgan, M. Crişan, M. Răileanu, D. Crişan, A. Ianculescu, P. Oancea, S. Şomăcescu, L. Todan, N. Stănică, B. Vasile, The effect of Co dopant on TiO2 structure of sol-gel nanopowders used as photocatalysts. Ceram. Int. 40, 12273–12284 (2014)
C. Khurana, O.P. Pandey, B. Chudasama, Synthesis of visible light-responsive cobalt-doped TiO2 nanoparticles with tunable optical band gap. J. Sol–Gel. Sci. Technol. 75, 424–435 (2015)
Alamgir, W. Khan, S. Ahmad, M.M. Hassan, A.H. Naqv, Structural phase analysis, band gap tuning and fluorescence properties of Co doped TiO2 nanoparticles. Opt. Mater. 38, 278–285 (2014)
A. Kaushik, B. Dalela, S. Kumar, P.A. Alvi, S. Dalela, Role of Co doping on structural, optical and magnetic properties of TiO2. J. Alloys Compd. 552, 274–278 (2013)
I. Ganesh, A.K. Gupta, P.P. Kumar, P.S.C. Sekhar, K. Radha, G. Padmanabham, G. Sundararajan, Preparation and characterization of Co-doped TiO2 materials for solar light induced current and photocatalytic applications. Mater. Chem. Phys. 135, 220–234 (2012)
F. Pacheco. M. González, A. Medina, S. Velumani, J.A. Ascencio, Structural analysis of cobalt titanate nanoparticles obtained by sol–gel process. Appl. Phys. A 78, 531–536 (2004)
S. Hon Lim, C. Ferraris, M. Schreyer, K. Shih, J.O. Leckie, T.J. White, The influence of cobalt doping on photocatalytic nano-titania: crystal chemistry and amorphicity. J. Sol. State Chem. 180, 2905–2915 (2007)
R. Rahimi, E. Honarvar Fard, S. Saadati, M. Rabbani, Degradation of methylene blue via Co–TiO2 nano powders modified by meso-tetra(carboxyphenyl)porphyrin. J. Sol–Gel. Sci. Technol. 62, 351–356 (2012)
K. Karthik, S. Kesava, K. Pandian, N. Suresh Kumar, Victor Jaya, Influence of dopant level on structural, optical and magnetic properties of Co-doped anatase TiO2 nanoparticles. App. Surf. Sci. 256, 4757–4760 (2010)
S.S. Babu, C. Mohandass, A.S.V. Raj, R. Rajasabapathy, M.A. Dhale, Multiple approaches towards decolorization and reuse of a textile dye (VB-B) by a marine bacterium Shewanella decolorationis. Water Air Soil Pollut. 224, 1500 (2013) (12 pp)
A.Y. Choi, C.-H. Han, Comparison of doping limits among sonochemically prepared metal-doped TiO2 nanopowders in view of physicochemical properties. Res. Chem. Intermed. 39, 1563–1569 (2013)
G. Westin, K. Jansson, A. Pohl, M. Leideborg, All alkoxide sol–gel route to CoO–TiO2 nano-powders. J. Sol–Gel. Sci. Technol. 31, 25–29 (2004)
K. Thamaphat, P. Limsuwan, B. Ngotawornchai, Phase characterization of TiO2 powder by XRD and TEM. Kasetsart J (Nat Sci) 42, 357–361 (2008)
P. Scherrer, Bestimmung der Grosse und der inneren Struktur von Kolloidteilchen mittels Rontgenstrahlen. Nachr. Ges. Wiss. Gott. 26, 98–100 (1918)
J.I. Langford, A.J.C. Wilson, Scherrer after sixty years: a survey and some new results in the determination of crystallite size. J Appl Cryst 11, 102–123 (1978)
S. Maensiri, P. Laokul, S. Phokha, A simple synthesis and magnetic behavior of nanocrystalline Zn0.9Co0.1O powders by using Zn and Co acetates and polyvinyl pyrrolidone as precursors. J Magn Magn Mater 305, 381–387 (2006)
D. Reyes-Coronado, G. Rodríguez-Gattorno, M.E. Espinosa-Pesqueira, C. Cab, R. de Coss, G. Oskam, Phase-pure TiO2 nanoparticles: anatase, brookite and rutile. Nanotechnology 19, 145605 (2008) (10 pp)
Y.-Q. Li, S.-G. Mei, Y.-J. Byon, J.-L. Wang, G.-L. Zhang, Highly solar radiation reflective Cr2O3–3TiO2 orange nanopigment prepared by a polymer-pyrolysis method. ACS Sustainable Chemistry& Engineering 2, 318–321 (2014)
J. Zou, W. Zheng, TiO2@CoTiO3 complex green pigments with low cobalt content and tunable color properties. Ceram. Int. 42, 8198–8205 (2016)
H. Tang, H. Berger, P.E. Schmid, F. Lévy, G. Burri, Photoluminescence in TiO2 anatase single crystals. Solid State Commun. 87, 847–850 (1993)
S. Mugundan, B. Rajamannan, G. Viruthagiri, N. Shanmugam, R. Gobi, P. Praveen, Synthesis and characterization of undoped and cobalt-doped TiO2 nanoparticles via sol-gel technique. Appl Nanosci 5, 449–456 (2015)
B. Santara, P.K. Giri, S. Dhara, K. Imakita, M. Fujii, Oxygen vacancy-mediated enhanced ferromagnetism in undoped and Fe-doped TiO2 nanoribbons. J Phys. D 47, 235304 (2014) (14 pp)
N.H. Hong, A. Barla, J. Sakai, N.Q. Huon, Can undoped semiconducting oxides be ferromagnetic?. Phys Status Sol C 4, 4461–4466 (2007)
Q. Xu, H. Schmidt, S. Zhou, K. Potzger, M. Helm, H. Hochmuth, M. Lorenz, A. Setzer, P. Esquinazi, C. Meinecke, M. Grundmann, Room temperature ferromagnetism in ZnO films due to defects. Appl. Phys. Lett. 92, 082508 (2008) (3 pp)
A.H. Farha, S.A. Mansour, M.F. Kotkata, Structural, optical, and magnetic study of dilute magnetic semiconducting Co-doped ZnO nanocrystals synthesized using polymer-pyrolysis route. J. Mater. Sci. 51, 9855–9864 (2016)
A. Sundaresan, C.N.R. Rao, Ferromagnetism as a universal feature of inorganic nanoparticles. Nano Today 4, 96–106 (2009)
D. Kim, J. Hong, Y.R. Park, K.J. Kim, The origin of oxygen vacancy induced ferromagnetism in undoped TiO2. J. Phys. 21, 195405 (2009) (4 pp)
A.K. Rumaiz, B. Ali, A. Ceylan, M. Boggs, T. Beebe, S.I. Shah, Experimental studies on vacancy induced ferromagnetism in undoped TiO2. Solid State Commun. 144, 334–338 (2007)
C.B. Fitzgerald, M. Venkatesan, J.G. Lunney, L.S. Dorneles, J.M.D. Coey, Cobalt-doped ZnO: a room temperature dilute magnetic semiconductor. Appl. Surf. Sci. 247, 493–496 (2005)
J.M.D. Coey, M. Venkatesan, C.B. Fitzgerald, Donor impurity band exchange in dilute ferromagnetic oxides. Nat. Mater. 4, 173–179 (2005)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mansour, S.A., Farha, A.H. & Kotkata, M.F. Sol–gel Synthesized Co-Doped Anatase TiO2 Nanoparticles: Structural, Optical, and Magnetic Characterization. J Inorg Organomet Polym 29, 1375–1382 (2019). https://doi.org/10.1007/s10904-019-01102-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10904-019-01102-6