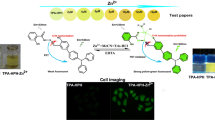
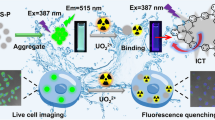
Abstract
ZnO nanoparticles (NPs) were prepared and characterized by different analytical methods and then they were used to decorate with N, N´-bis(salicylidene)ethylenediamine (salen) in order to perform as receptor for the metal ions in an aqueous medium. The results show that ZnO-salen selectively detects Al3+ ions in aqueous medium since the intensity of fluorescence has been enhanced significantly. However, the presence of K+ in the medium further intensified the fluorescence emission for the [ZnO-salen-Al3+] system. The above system has been applied to recognize Al3+ and K+ in cells by developing the cell images, for which, the fluorescence image is brightened if a human glioblastoma U251 cell contains [ZnO-salen-Al3+] + K+ ions, consisting of the fluorescence titration. The binding global constant for Al3+ and the subsequent recognition of K+ by ZnO-salen resulted in β2(Al3+) = 6.61 × 103 and β2(K+) = 3.71 × 103 with a detection limit of 36.51 µM for Al3+ and 17.39 µM for K+. In the cell toxicity analysis, the cell viability was over 85% for the ZnO-salen even in the concentration as high as 100 mM.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Highly selective fluorescent sensors are interesting in the view of detection and quantification of various metal ions or anions in the biological and environmental samples [1]. So, the development of such molecular devices is attractive since their distinct optical properties are of great interest and the specific interaction of fluorophores (host or receptor) with analytes (guests = cations or anions) can undergo a substantial conformational change in order to enhance or suppress the florescence intensities; i.e. the binding of receptor-analytes forms Host-Guest type of complex that behaves spectrally or electrochemically different from those of individual (receptor or guest) moiety [2]. Thus, several researchers are interested to develop molecular recognition/sensing systems for the specific recognition of cation or anion, or neutral organic molecules in analytical/biological/environmental samples [3]. However, sometime, it involved several steps to synthesis selective chemosensor having pseudo cavities compatible with analyte as it requires long synthetic skills [4]. Additionally, the efficiency of molecular recognition depends on many factors, of which solvent medium plays a vital role for the formation of hydrogen bonds between host molecule and guest moiety, and it develops a signal depending on the variation of the size and shape of host or guest moieties [5]. The nature of ligand attached to the binding site, and the pH of the medium are being considered essentially in the recognition process [6]. The whole issue mostly is associated with hydrophobic or hydrophilic nature of polar solvents [7].
The development of real-life materials requires robust systems, ideally, full aqueous systems would be desirable from the environmental point of view. The fluorescent response strongly depends on the energy transfer efficiency, and it is directly related to the physical distance between nanomaterials and guest ions [8,9,10,11]. As many organic chemosensors have a limited solubility in water, but it is required to be used at the same time many in aqueous matrices where relevant ions are found to be presented. Thus, it is obligated to disperse the mentioned chemosensors in aqueous systems, so it can be converted to Organic Nanoparticles (ONPs) which turned to be a stable colloidal suspension giving organic nano clusters with high surface area and great sensing potential. Thus, the Organic nanoparticles (ONPs) have been extensively investigated due to their potential use in medicine as drug delivery systems [12, 13]. To further increase selectivity and recognition properties of ONPs, the strategy is to restrict some of the coordination modes, limiting the flexibility of the free organic ligand through the bonding of the ONPs with metal or metal oxide/sulfide surfaces [14, 15]. In contrast, free ligands are highly flexible and can adopt multiple geometry depending on the steric requirement of a given metal ion. It is known that the ability of the coordination of N, N´-bis(salicylidene)ethylenediamine (salen) with different metallic ions including Cu2+, Ni2+, Zn2+, etc. is well established [16, 17]; however, this ability can be tuned through the immobilization agent and the composition of the supporting material. Moreover, the presence of metallic clusters such as Cu, Ag and Au in the system has further promoted the fluorescent response due to the overlapping of the d-orbitals with the orbital from the imine group that could support otherwise forbidden relaxation-emissive transitions [18]. For example, the decoration of a non-selective organic receptor over the surface of CdSe/ZnS (QDs) showed a unique selectivity towards cations and this ability was employed for quantification and bioimaging in biological or environmental matrices [19]. Also, solvent and pH could influence and tune recognition properties, for example, ZnO immobilized imines have been utilized for the selective recognition of Mg2+ [20] and substitution over aromatic rings in salen-like ligands can also modify and enhance fluorescent response towards specific ions and biomolecules [21, 22]. Thus, there is a high scientific interest on fine-tuning sensing, both to understand transfer mechanisms and for applications such as bio and chemosensors and devices based on logic gates optical memory [23].
When compared to the traditional organic fluorophores, quantum confined nanoparticles are a new class of probes that exhibit more unique optical characteristics (high quantum yield, good photostability and large Stokes shift) which have been widely exploited in the area of biosensing, immunoassays, and biological imaging [23]. Therefore, the construction of nanocomposites, composed of ONPs, and metal and metal oxides might provide additional tools to visualization by conventional microscopy in cellular imaging due to their excellent and stable fluorescence properties. It can also help in solving the transport problems for pharmacologically relevant compounds that need to be internalized. On the other hand, the thermodynamic stability of metal, metal oxides or metal chalcogenides nanoparticles can be often achieved by chelating of organic molecules on the surface of the nanoparticle [24,25,26,27]. For example, citrate or ascorbate can act as a reducing and also as capping /stabilizing agent for the metal nanoparticles. For the preparation of AuNPs, (i) citrate or ascorbate reduction [28,29,30], (ii) the stabilization by thiols [31,32,33,34,35], (iii) the reducing agents such as NaBH4 [31], white phosphorus [36], ethanol, and polyols are employed [37]. The advantage of trisodium citrate dehydrate or ascorbate reduces the metal ions, forming metal NPs efficiently, and it also stabilizes the size of the NPs by decreasing the large nanoparticle interface energy. However, salen is a good capping agent due to its chelating capability that can stabilize NPs efficiently; in addition, it has two imine groups, involving the bonding with metal NPs through its conjugated π electrons that support the photophysical and photochemical properties.
The ONPs of salen have been coated with metals (Ag0 or Au0), metal oxides (ZnO, CdO) or chalcogenides (ZnS, CdS) and observed the biological activity dependence with respect to the size, shape and supramolecular organization of the composites. Although there are several organic molecules based chemo-sensors for the recognition of Al3+ [38,39,40,41,42,43,44,45,46,47,48,49,50], the metal oxide nanoparticles bounded with organic molecules for the detection of Al3+ are limited in literature [51,52,53,54], in particular, the development of human cell images by ZnO based sensors in aqueous medium is very limited; thus, we have focused on ZnO bounded with N, N´-bis(salicylidene)ethylenediamine (salen) for the recognition of Al3+ in the human cells. Several studies have shown that internalization of these NPs is possible and can be, therefore, employed potentially as real time sensors in living matrices; thus, it has become a key research topic. To assess the applicability of salen ligand as a fluorescent chemosensor, in the present work, we are testing the recognition capacities and toxicological potential of metal oxides (ZnO) coated with ONPs. The most robust recognition system was further studied in its recognition capabilities under different solvents systems and applied in the real time bioimaging using U251 human cells.
Experimental
General Information
All analytical grade chemical reagents and solvents were purchased from Millipore-Sigma and used as received from the company without any further purification.
Instrumentation
All UV–Vis absorption spectra are recorded on a Perkin Elmer Lambda 25 and fluorescence studies were performed on a F96 Pro. For FTIR, a Shimadzu IRTracer 100 with a QATR-10 accessory was used. The Transmission electron microscopy (TEM) images were obtained on a JEOL 2010 (200 kV) using 200 mesh C-coated copper grids. An Advance Davini 8 instrument was employed to obtain XRD patterns (CuKa = 0.1254 nm). An Olympus CKX41, USA Inverted microscope was used in cell viability and bioimaging studies; for nonlinear regression studies in cell culture GraphPad Prism 6.0e was utilized (GraphPad Software, Inc., USA).
Synthesis of ZnO-Salen NPs
The experimental details of the above material preparation are illustrated in Scheme 1 and explained as follows:
Salen
The Salen ligand (N, N´-bis(salicylidene)ethylenediamine) was synthesized by using the procedure published elsewhere [55]. To a solution of ethylenediamine (0.60 g, 1.0 mM) dissolved in EtOH (8.0 mL), salicylaldehyde (0.244 g, 2.0 mM) dissolved in EtOH (12 mL) was slowly added, and the resulting solution mixture was stirred for 30.0 min at room temperature. The yellow crystalline solid obtained was filtrated and washed several times with diethyl ether. Yield was found to be 87%. Elemental analysis for C16H16N2O2, C, 71.62; H, 6.01; N, 10.44; O, 11.93. Found: C, 71.94; H, 5.64; N, 10.85. 1H NMR (300 MHz, CD3OD): 2CH2-N (4 H, d, 3.95), aromatic H (4 H, m, 6.84–6.82), aromatic H (4 H, m, 7.30–7.25), 2 N = CH (2 H, s, 8.44). 13C NMR (75 MHz, CD3OD): 166.87, 161.80, 132.28, 131.47, 118.04, 116.55, 58.59. IR: 3208 (-OH), 3047 (-CH2-), 1910 (-Ph), 1698 (-C = N-).
Salen ONPs
Organic Nanoparticles (ONPs) were prepared by a 1.0 mL of a 0.1 mM solution of Salen in THF was slowly injected (0.5 mL/min) with a peristaltic pump into 100 mL of distilled water using a 6 mm silicon tube and a 30 mm stainless steel needle under sonication (Scheme 1). The salen ONPs was characterized with TEM and X-ray diffraction pattern.
ZnO-Salen NPs
ZnO was prepared by a hydrothermal method (V = 70 mL,180 °C, 12 h) using a solution of Zn(NO3)2·6H2O (595 mg, 2.0 mmol) and NaOH (120 mg, 3.0 mmol) dissolved in ethanol. After the reaction a white product was separated out, washed with ethanol, and dried at 105 °C, 12 h. Dry ZnO (10 mg/L) was dispersed by sonication in distilled water. To this dispersion of ONPs were carried out by injecting 1.0 mL of a THF solution of Salen precursor as mentioned above. The ZnO-salen NPs was characterized with TEM and X-ray diffraction pattern.
Metal ion Recognition Studies
The recognition of metal ions by ZnO-salen was performed by measuring the fluorescent change in the emission of the sensor system in the presence of 1.0 mM solutions containing different nitrate salts (Na+, Mg2+, Al3+, K+, Cr3+, Mn2+, Fe3+, Co2+, Ni2+, Cu2+, Zn2+, Sr2+, Ru3+, Ag+, Cd2+) in a full aqueous environment provided by regular distilled water (pH 7.0; HEPES 0.5 M). Additionally, the changes in the detection accuracy were studied in different MeCN-H2O solvent systems to give an insight into the robustness of the method towards complex media typically observed in industrial waste streams.
Effect of pH and Temperature
The change of the fluorescent response for salen ONPs and ZnO-salen NPs was studied under different pHs, and also different temperatures in order to explore its potential application in harsh environments. ZnO-salen NPs or salen ONPs (50 mL, 0.5 mM) were suspended in water, and measured the intensity of fluorescence emission at 450 nm or 435, respectively; the effect of pH (from 3.0 to 11.0) was studied, measuring the fluorescence intensity after successive addition of HNO3 (0.1 M) or NH4OH (0.1 M); similarly, the temperature effect (20 to 80 °C, dT < 10°/min) with the intensity was also recorded.
Sensing Recognition Studies
Whenever a system shows a positive response towards certain particular recognition for the developed ZnO-salen NPs, the stability was tested through a titration and interference test. First, the change of fluorescence intensity of the ZnO-salen NPs is measured upon adding the selected metal ion (Mn+); the concentration (C) is progressively increased until the changes in emission (E) cease, and the system stabilizes (dE/dC0). To determine stoichiometry for ZnO-salen NPs with Mn+ binding, a Job test was conducted where the molar fraction of the ZnO-salen NPs-Mn+ system is kept constant, but the molar fraction of each component is varied, and the fluorescent response is determined. The maximum change in the fluorescence at a particular molar fraction would, therefore, correspond to the highest host-guest interaction.
Toxicity by Cell Viability Studies
The human glioblastoma cell line U251 (ATCC, USA) was used to evaluate the cell toxicity of ZnO-salen NPs. Cells were grown with high-glucose DMEM medium (Biowest, USA) supplemented with 10% fetal bovine serum, 1.0 mM pyruvate and 0.1 mM non-essential amino acids (Biowest, USA) at 37 oC under a 95% air and 5% CO2 atmosphere. 25 × 103 cells were seeded in 6-well plates and treated with different concentrations (1.0 nM to 100 µM) of salen ONPs (used as vehicle), and ZnO-salen NPs, and after 24 h the cells were harvested with 1.0 mL phosphate saline buffer (PBS) with 1.0 mM EDTA and counted by a blind observer in a Neubauer chamber. Cell viability was also measured with the trypan blue dye exclusion test. The percentage of cells obtained from the ZnO-salen NPs treatment was plotted and the half maximal inhibitory concentration (IC50) was calculated with a non-linear regression analysis.
Bioimaging Studies
The recognition analysis was performed by measuring the changes in fluorescent emission Human cells were cultured as previously described and 100 µL of this culture were inoculated onto glass slides to which 10 µL of the desired compound was added and a stabilization time of 30 min was established. Samples were thus observed in an Olympus FV1000 equipped with a 200–1000 nm lamp.
Results and Discussion
Characterization of Materials
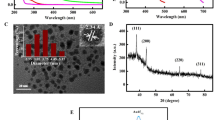
The morphology and size of prepared sensor were analyzed by electron microscopy and Dynamic Light Scattering and observed that ZnO-salen NPs are composed of agglomeration of ZnO particles with size above 200 nm; it is noticeable that a surface coating of a low-density material was seen identifying as salen ONPs (Fig. 1a). The DLS results show that salen ONPs are composed of colloidal particles having classified in three main groups based on its size: from 15 to 40 nm with maximum population (Pmax) at 32 nm (16.3%); from 55 to 100 nm (Pmax=37 nm), accounting for 29.6% and from 65 to 160 nm (Pmax=91 nm) representing 54.4%. As bigger particles are formed, they tend to stabilize, thus being the main components of the colloidal particles. Prior to salen ONPs deposition size, zeta potential (ζ) of bare ZnO was determined. ZnO was composed of a single group of particles with size ranging from 122 to 340 nm with Pmax=220 nm (31%); dispersion stability was among the reported values being ζ =+27 mV. Upon salen ONPs formation, ZnO-salen NPs was mostly found to be agglomerated around Pmax=712 ± 200 nm, 83%. There was a minor population (13%) at Pmax= 190 nm that was identified as uncoated ZnO particles and the average ζ of this material was + 19 mV (Fig. 1b). To confirm the formation of ZnO from precursors, XRD pattern was determined after hydrothermal treatment and the main peaks for wurtzite were identified at 31.7 [100], 34.4 [002], 36.1 [101], 46.9 [102], 56.4 [110], 62.8 [103], 64.5 [200], 67.9 [112], 69.4 [201], 74.1 [004] and 77.8 [202]. After deposition of ONPs, the peaks from ZnO were conserved and observing the emergence of a new peak around 28.2° that has been previously reported in Salen-doped metal oxides (Fig. 1c).
The emission spectra of pure salen ONPs at different excitation wavelengths exhibited a maximum band at 435 nm; however, the best fluorescence intensity response has been seen for an excitation wavelength at 365 nm (Fig. 2a). On the other hand, the fluorescence emission spectrum for ZnO-salen NPs (0.5 mM) was resulted exhibiting an enhanced blue emission band at 450 nm (λex = 365 nm), because of the coating of salen ONPs on the surface of ZnO NPs (Fig. 2b).
Effect of pH and Temperature on the Fluorescent Response
The fluorescence intensity of salen ONPs and ZnO-salen NPs was measured (I/I0 = 1.0 at pH 6.5 and 20 °C) at different pHs, showing the enhancement of the emission intensity in basic condition (at high pH), and the quenching of the emission in acidic medium (low pHs) (Fig. 3). For example, for salen ONPs, a maximum intensity has reached at pH 9.1 (I/I0 = 3.19), and for ZnO-salen NPs, it maintains a high emission intensity (an average of I/I0 = 1.1) from pH 7.0 to 11.0. In contrast, under acidic condition, a low fluorescence emission was observed (pH 3.6, I/I0 = 0.2 for salen ONPs, and I/I0 = 0.12 for ZnO-salen NPs) due to the protonation of imine nitrogen. This observation is consistent with the reported studies [56, 57], especially, for ZnO-based material [58] and ZnO-imine composites [20, 59].
The temperature change with the fluorescence was also monitored, and showed that after 45 °C, there was a significant quenching of fluorescence for salen ONPs in contrast to ZnO-salen NPs, where there is a considerable enhancement of fluorescence was seen (Fig. 3). For example, for salen ONPs, the temperature from 20 to 39 °C, the intensity was reached to I/I0 = 1.64, after which, a dramatic decrease (I/I0 = 0.62 at 74 °C) was noted. For ZnO-salen NPs, the emission was increased to I/I0 = 2.06 at 57 °C, and then, it was gradually decreased to I/I0 = 1.82 at 74 °C. This is probably due to the evaporation of solvent from the mesoporous structure of ZnO at high temperature, and it allows its active sites for the interaction of salen ligand. However, at high temperature, it is highly possible that imine groups can undergo a hydrolysis, affecting the fluorogenic activity.
Cationic Recognition Tests for Sequential Selective Sensing of Al3+ and K+ by ZnO-Salen NPs
In the cationic recognition study, ZnO-salen was employed as chemosensor for the recognition of Al3+, and observed a significant florescence change only for Al3+ after testing several metal ions (Na+, K+, Mg2+, Sr2+, Al3+, Cr3+, Mn2+, Fe3+, Ru3+, Co2+, Ni2+, Cu2+, Ag+, Zn2+, and Cd2+) (Fig. 4b). For the samples, the solution was excited at 365 nm and the fluorescence emission was observed in the visible region. This same test was also performed for salen ONP; however, no selectivity was observed after using the same cations in the form of nitrate salts (Fig. 4a). Additionally, an identical test was performed for anions using ZnO-salen NPs as chemosensor, but no such changes were found after using the following anions in the form of tetrabutylammonium salts (F-, I-, Cl-, Br-, HSO4-, ClO4-, AcO- and H2PO4-). Thus, this ZnO-salen system is suitable only for Al3+ (Fig. 4b), consisting with the titration of Al3+ vs. ZnO-salen NPs (Fig. 5), where for the successive addition of Al3+ to ZnO-salen NPs (0.1 mM), an enhancement of fluorescence intensity at 490 nm was observed. This means that the binding nature of ZnO-salen (receptor) with Al3+ enhances the fluorescence intensity. This can be explained as follows:
-
(i)
The addition of Al3+ to the solution of ZnO-salen undergoes a red-shift in the peak, probably due to strong coordination of Al3+ ion with ZnO-salen during the high concentration of Al3+ in the solution. The PET tuned to ‘ON’ from the OH if any metal ion binds to receptor by using the OH binding site, and it increases the fluorescence intensity on the same wavelength. Nevertheless, if any metal ion also binds to CH = N, a close binding to fluorophore leads to the shift the wavelength position because of changing in the modulation of transfer charge transfer.
-
(ii)
The excitation of ZnO-salen without the Al3+ presence, the energy level of highest occupied molecular orbital (HOMO) of the receptor (before binding with ZnO-salen) would be higher compared to that of the excited fluorophore where HOMO would have half-filled. So, this electronic energy configuration encourages a rapid electron transfer from the receptor to the excited-state fluorophore, and it favors a quenching of fluorescence intensity. Whereas, if the interaction of ZnO-salen (ionophore) with Al3+, the receptor’s HOMO energy level is turned to be lower, inhibiting the receptor electron transfer to the HOMO of excited-state fluorophore, and it causes the fluorescence mode to be “Turn-On”.
After establishing Al3+ recognition capacity by ZnO-salen NPs, Job’s plot measurements based on the emission were performed. The emission has exhibited at 490 nm with a maximum when the mole fraction was 0.4 (Fig. 5c), which belongs to a 1:2 binding stoichiometry for the system of ZnO-salen NPs with Al3+. The Fig. 5a illustrates the family of emission spectra obtained when an aqueous solution (HEPES, 0.5 M at pH = 7.0) of ZnO-salen NPs (0.1 mM) is titrated with Al3+ ions (0–20 mM) and the Fig. 5b shows the ratiometric response of intensity at 490 nm/450 nm on progressive addition of Al3+. The profile curve of the ratiometric change of emission at 490 nm/450 nm remained unchanged when the concentration of Al3+ was 20 mM. The titration profile showed a sigmoidal curve indicating the formation of two-step complexation process. This profile could be well fitted to a 2:1 (ZnO-salen: Al3+) binding model using a nonlinear least-squares treatment with Eq. (1) [60, 61] to give a global formation constant of β2 = K1·K2 = 6.61 × 103 ± 0.04, where K1 and K2 are the complexation constants for the first and second Al3+ atom, IF is the observed intensity at 490 nm and I1 and I2 are the emission intensity of the 1:1 and 1:2 stoichiometry, respectively. The Job’s plot confirmed this stoichiometry (Fig. 5c).
a) Spectrofluorimetric titration of buffered aqueous solution (0.5 M, HEPES at pH 7.0) of ZnO-salen NPs (0.1 mM) by Al3+. The arrows show the direction of the spectral changes. b) Profile of emission at 490 nm for increasing amounts of Al3+ (average of triplicate experiments). The line was obtained by fitting the data to a 2:1 (Al3+:ZnO-salen) binding model using Eq. (1). c) Stoichiometric analysis of ZnO-salen NPs by Job’s plot with Al3+ at pH = 7.0
Notably, there is a linear dependence in the fluorescence intensity for ZnO-salen NPs with Al3+ at different concentrations (range of 0–6.0 mM, R2 = 0.99832) in an aqueous solution, and it gives a limit of detection (LOD) of 36.51 µM. The LOD was calculated using the formulae LOD = 3σ/s, where σ = standard deviation of blank luminescence intensity and s = slope of the calibration plot, s = 13.15 (± 0.18) × 103 M (Figure S4). For practical applications, the chemosensors must have not only good optical response and affinity, but also selectivity in the presence of coexistent potential interferences in real samples. Therefore, a selectivity experiment of ZnO-salen-Al3+ (0.1 mM ZnO-salen + 0.2 mM Al3+) towards the possible interfering metal cations was carried out at pH = 7.0. The addition of these cations to aqueous suspensions of ZnO-salen NPs has produced a negligible change in its emission, except for the addition of K+, for which, an enhancement of fluorescence intensity was observed (Fig. 6).
To learn more about the properties of ZnO-salen-Al3+ as a receptor for K+, fluorescence titration was carried out (Fig. 7a). The addition of increasing amounts of potassium to the suspension of ZnO-salen-Al3+ has further increased the emission intensity at 490 nm, which was attributed to the formation of an ZnO-salen-Al3+-K+ complex with a β2 = 3.71 × 103 (Fig. 7b, Eq. 1) and a host guest ratio of 1:2 determined from Jobs plot in aqueous environment (HEPES, 0.5 M at pH = 7.0) (Fig. 7c). There is a linear dependence in the fluorescence intensity for ZnO-salen-Al3+ with K+ at different concentrations (range of 0–7.0 mM, R2 = 0.9987) in an aqueous solution, and it gives a limit of detection (LOD) of 17.39 µM (Figure S5). The observed fluorescent recognition results could be explained as follow: The stepwise addition of K+ to ZnO-salen-Al3+ leads to the formation of intramolecular coordination sphere using both O-H and C = N linkages, thus PET is cancelled from O-H and Charge Transfer band is also affected.
a) Spectrofluorimetric titration of buffered aqueous solution (0.5 M, HEPES at pH 7.0) of ZnO-salen-Al3+ (0.1 mM) by K+. The arrows show the direction of the spectral changes. b) Profile of emission at 490 nm for increasing amounts of K+ (average of triplicate experiments). The line was obtained by fitting the data to a 2:1 (K+:ZnO-salen-Al3+) binding model using Eq. (1). c) Stoichiometric analysis of ZnO-salen-Al3+ by Job’s plot with K+ at pH = 7.0
Additionally, the relative quantum yields of ZnO-salen NPs with Al3+ and K+, in HEPES buffer at pH 7.0 were calculated using quinine sulfate in 0.1 M H2SO4 as the reference (Φref = 0.540) according to the Eq. (2), where the subscripts “x” and “ref” denote sample and reference standard. F is the spectrally integrated photon flux at the detector, i.e., the area under the emission spectrum, and A is absorption factor provides the fraction of the excitation light absorbed by the sample and reference standard. The Eq. 2 contains also a refractive index correction term (η) that has to be applied if different solvents are used for sample and standard. The relative quantum yields of ZnO-salen, ZnO-salen-Al3+ and ZnO-salen-Al3+-K+ are Φ = 0.098, Φ = 0.109 and Φ = 0.120, respectively.
The relative quantum yields show that there is an enhancement of the emission intensity after the sequential addition of Al3+ and K+. This photophysical phenomenon is attributed to chelation-enhanced fluorescence (CHEF) mechanism operating between Al3+ and ZnO-salen; thus, it, subsequently, cancels the photoinduced electron transfer (PET) process, promoted by ion-dipole- electrostatic interaction that presented between potassium ions and salen ligand (imine and phenol groups) [62]. According to fluorimetric titrations, it follows a turn-on fluorescent detection mechanism as it consists with job plots and the fluorescence quantum yields (Scheme 2).
Logic gates for CDs are important for detecting various analytes and generally most of the logic gates are fundamentally based on fluorescence spectroscopy because of their sensitiveness. According to the results, it was found that a logic gate system exists in the recognition of K+ by ZnO-salen in presence of Al3+ ions. This means that the enhancement of fluorescence intensity for K+ with ZnO-salen NPs was observed only if the Al3+ ions are presented; however, the intensity was low if other combinations of ZnO-salen NPs with K+ are used. First, an AND logic gate was performed through the binding of ZnO-salen NPs with Al3+ ions [A = 1, B = 1], turning on the fluorescence was seen at 490 nm. In another AND logic gate [A = 1, B = 1, C = 1], the fluorescence intensity of ZnO-salen-Al3+ increased when K+ ions are added. This indicates that ZnO-salen NPs first recognize Al3+ ions and then detect K+ ions (Scheme 3).
Toxicity Studies of ONPs in Human U251 Cells
The human glioblastoma derived cell line U251 was used for the cytotoxic evaluation of different salen ONPs coupled to various metals (ZnO, Au0, and Ag0). For this purpose, different concentrations of the salen ONPs were used (1 nM to 100 µM) and the number of cells as well as their viability was evaluated. Salen ONPs and Au-salen NPs slightly decreased the number of cells in a concentration-dependent manner, while Ag-salen NPs significantly reduced it at 100 µM. Interestingly, ZnO-salen NPs did not reduce the number of cells, and on the contrary, it significantly increased it as compared with the salen ONP treated cells from 10 µM to 100 µM (Fig. 8a). Despite the effects on the number of cells, the cell viability was maintained (85–90%) for salen ONP, ZnO-salen NPs, and Au-salen NPs treatments. However, Ag-salen NPs markedly reduced cell viability to 3% when the highest concentration was used (100 µM) (Fig. 8b). These data suggest that high concentrations of ZnO-salen NPs increase the proliferation capabilities of the cells without altering their viability, while Ag-salen NPs promotes cell death through a cytotoxic effect. Once the cytotoxic effect of Ag-salen NP was evaluated, the data were used to calculate the half maximal inhibitory concentration, IC50 = 20 µM according to the dotted line representing the non-linear regression model shown in Figure S6. It is suggested that the acidic environment of the lysosomes promotes the degradation of the ONP coating with the subsequent release of the metal and metal oxide particles. In case of Ag and Au clusters, they oxidize into toxic metal ions, although their release depends on the coating structure [63, 64]. Once inside the cell, metal ions can disrupt the cell membranes, generate reactive oxygen species (ROS), damage protein’s structure and function, and disrupt the DNA structure, leading eventually to cell death [65].
Our results show that 100 µM Ag-salen NPs reduces the number and the viability of U251 cells. Elemental silver (Ag0) can be oxidized into Ag+ under physiological conditions which is known to have toxic effects in the human colorectal cancer cells Caco-2 [66] and reduce cell viability and promote cell death in the human myeloid leukemia cell line SHI-1 [67]. In the glioblastoma cell line U87 coated Ag nanoparticles induce DNA damage, increase oxidative stress, mitochondrial dysfunction, and cell death [68]. The reduced cell viability observed for the U251 cells should be through Ag+ accumulation inside the cell and DNA fragmentation that eventually leads to cell death. In case of salen ONPs and Au-salem NPs, there is a tendency to reduce the number of cells in a concentration-dependent manner without affecting the cell viability. This suggests a possible cell cycle arrest and cell toxicity could emerge only under very high concentrations. Interestingly, treatment with 10 and 100 µM of ZnO-salen NPs increased the number of cells. ZnO nanoparticles are known to induce ROS production in the cells [69], and reports show that in mouse macrophages ZnO nanoparticles uptake elicit a cytotoxic response that does not depend on the cellular increase in ROS [70]. In contrast, in human myoblastoma cancer cells, ZnO nanoparticles increase ROS production and caspase-3 activity thus promoting cell death [71]. However, a low level of ROS generation promotes the proliferation of several types of cancer cells and, remarkably, the overexpression of mitochondrial protein Romo1 in glioblastoma cells is associated with ROS production and cell proliferation [72, 73]. Therefore, ZnO-salen NPs internalization could be promoting an increase in ROS production that should be favoring U251 cell proliferation.
Effects of salen ONPs based materials concentration (1.0 nM to 100 µM) on U251 cell number and viability upon 24 h treatment: a) number of cells and b) viable cells after treatment with the different concentrations (log M). *P < 0.05 vs. salen ONP and Ag-salen NPs, **P < 0.01 vs. all other groups, mean ± S.E.M., n = 4
Application of ONP-ZnO in Bioimaging
From Fig. 9, it can be observed that fluorescent emission within cells upon excitation at ~ 490 nm follows the trends previously observed in aqueous solution, which indicated that the interaction between ZnO-salen NPs and analytes is strong enough to preserve its activity in biological media, where multiple anions and cations are usually present. ZnO-salen NPs that are present in the cell culture are internalized through endocytosis and stored in lysosomal compartments. In presence of ZnO-salen NPs, fluorescence displayed is mainly centered in the nucleus and its boundaries while in presence of Al3+, fluorescence is extended to plasmatic region. These changes can be explained since the cytosol is a region where Al3+ ions can be freely dispersed and thus interaction with ZnO-salen NPs could take place before this system is attached to nucleus borders. For addition of ZnO-salen NPs and K+, no fluorescence was observed at all, but in case of sequential addition of Al3+ and K+, a slight enhancement in fluorescence intensity is observed; also, the emission is observed uniformly distributed among cytosol which confirms that dispersed ions in cytosol contribute to interact with ZnO-salen NPs. It is important to observe that changes in fluorescence between ZnO-salen-Al3+ and ZnO-salen-Al3+-K+ are not as intense as observed in vitro; this is understandable since most living cells contain K+ and thus an instant interaction and interference would be expected towards freshly formed ZnO-salen-Al3+ [74, 75].
Conclusions
Salen Organic Nanoparticles and ZnO-salen hybrid were prepared and studied their photophysical and photochemical properties. The results reveal that ZnO-salen selectively recognizes Al3+ ions in aqueous medium by enhancing the fluorescence intensity. The effect of pH and temperature is also performed, showing an enhancement of fluorescence intensity at high pH, yielding a maximum emission at pH 9.1 for salen ONPs, and pH 9.7 for ZnO-Salen; at low pH, a quenching of the intensity is observed, due to the protonation of the ligand. With the increase of temperature, the increase of fluorescent intensity is noticed, namely, it has reached a maximum fluorescence at 39 °C for Salen ONPs, while for ZnO-salen, it was at 57 °C. With the presence of K+ in the medium, it is further intensified the emission for the [ZnO-salen-Al3+] system. The observed binding constants are β2(Al3+) = 6.61 × 103, and β2(K+) = 3.71 × 103 (subsequent recognition of K+ by ZnO-salen), and the limit of detection found to be 36.51 µM for Al3+ and 17.39 µM for K+. The above system is employed to recognize Al3+ and K+ in cells by developing the cell images for glioblastoma U251, and observed the brightening of the image for [ZnO-salen-Al3+] + K+ ions. Cell toxicity analysis shows the cell viability of 85% for the ZnO-salen.
Data Availability
No datasets were generated or analysed during the current study.
References
Yan K-C, Steinbrueck A, Sedgwick AC, James TD (2021) Fluorescent chemosensors for Ion and Molecule Recognition: the next chapter. Front Sens 2. https://doi.org/10.3389/fsens.2021.731928
Hamilton GRC, Sahoo SK, Kamila S et al (2015) Optical probes for the detection of protons, and alkali and alkaline earth metal cations. Chem Soc Rev 44:4415–4432. https://doi.org/10.1039/C4CS00365A
Wu D, Sedgwick AC, Gunnlaugsson T et al (2017) Fluorescent chemosensors: the past, present and future. Chem Soc Rev 46:7105–7123. https://doi.org/10.1039/C7CS00240H
Khan J (2023) Synthesis and applications of fluorescent chemosensors: a review. J Fluoresc. https://doi.org/10.1007/s10895-023-03455-1
Krämer J, Kang R, Grimm LM et al (2022) Molecular Probes, chemosensors, and Nanosensors for Optical Detection of Biorelevant Molecules and ions in Aqueous Media and Biofluids. Chem Rev 122:3459–3636. https://doi.org/10.1021/acs.chemrev.1c00746
Fu Y, Finney NS (2018) Small-molecule fluorescent probes and their design. RSC Adv 8:29051–29061. https://doi.org/10.1039/C8RA02297F
Xiang H, Cai Q, Li Y et al (2020) Sensors Applied for the detection of pesticides and Heavy metals in Freshwaters. J Sens 2020:1–22. https://doi.org/10.1155/2020/8503491
Wang X, Kong L, Zhou S et al (2022) Development of QDs-based nanosensors for heavy metal detection: a review on transducer principles and in-situ detection. Talanta 239:122903. https://doi.org/10.1016/j.talanta.2021.122903
Adam T, Gopinath CB S (2022) Nanosensors: recent perspectives on attainments and future promise of downstream applications. Process Biochem 117:153–173. https://doi.org/10.1016/j.procbio.2022.03.024
Sharma V, Tiwari P, Kaur N, Mobin SM (2021) Optical nanosensors based on fluorescent carbon dots for the detection of water contaminants: a review. Environ Chem Lett 19:3229–3241. https://doi.org/10.1007/s10311-021-01241-8
Liu B, Zhuang J, Wei G (2020) Recent advances in the design of colorimetric sensors for environmental monitoring. Environ Sci Nano 7:2195–2213. https://doi.org/10.1039/D0EN00449A
Pan K, Zhong Q (2016) Organic nanoparticles in Foods: fabrication, characterization, and utilization. Annu Rev Food Sci Technol 7:245–266. https://doi.org/10.1146/annurev-food-041715-033215
Sharma S, Singh A, Kaur N, Singh N (2022) Sensing of environmentally and biologically important analytes using organic nanoparticles (ONPs). Sensing and Biosensing with optically active nanomaterials. Elsevier, pp 365–399
Yadav A, Upadhyay Y, Bera RK, Sahoo SK (2020) Vitamin B6 cofactors guided highly selective fluorescent turn-on sensing of histamine using beta-cyclodextrin stabilized ZnO quantum dots. Food Chem 320:126611. https://doi.org/10.1016/j.foodchem.2020.126611
Dhanshri S, Sahoo SK (2023) Fluorescence turn-on detection of Alkaline phosphatase activity and Al3+ using vitamin B6 cofactor conjugated GSH capped Mn-doped ZnS Quantum dots. J Fluoresc 33:587–594. https://doi.org/10.1007/s10895-022-03051-9
Asatkar K, Tripathi A, Asatkar M D (2020) Salen and related ligands. In: Stability and Applications of Coordination Compounds. IntechOpen
Erxleben A (2018) Transition metal salen complexes in bioinorganic and medicinal chemistry. Inorganica Chim Acta 472:40–57. https://doi.org/10.1016/j.ica.2017.06.060
Bhardwaj V, Bothra S, Upadhyay Y, Sahoo SK (2021) Cascade Detection of Pyridoxal 5′-Phosphate and Al 3+ ions based on dual-functionalized red-emitting copper nanoclusters. ACS Appl Nano Mater 4:6231–6238. https://doi.org/10.1021/acsanm.1c01019
Singh N, Mulrooney RC, Kaur N, Callan JF (2008) A nanoparticle based chromogenic chemosensor for the simultaneous detection of multiple analytes. Chem Commun 4900. https://doi.org/10.1039/b813423e
Sharma H, Kaur N, Pandiyan T, Singh N (2012) Surface decoration of ZnO nanoparticles: a new strategy to fine tune the recognition properties of imine linked receptor. Sens Actuators B Chem 166–167:467–472. https://doi.org/10.1016/j.snb.2012.01.076
Sharma H, Singh A, Kaur N, Singh N (2013) ZnO-Based imine-linked coupled biocompatible Chemosensor for Nanomolar Detection of Co 2+. ACS Sustain Chem Eng 1:1600–1608. https://doi.org/10.1021/sc400250s
Kaur K, Kaur N, Singh N (2012) Imine coupled ZnO based fluorescent chemosensor for the simultaneous estimation of Al3+ and Cr3+. Mater Lett 80:78–80. https://doi.org/10.1016/j.matlet.2012.04.043
Kaur N, Bharadwaj VK, Kaur K, Singh N (2015) Surface decoration of Organic ligands on Quantum dots: fine tuning of Photophysical Properties. Handbook of nanoparticles. Springer International Publishing, Cham, pp 1–20
Jana NR, Gearheart L, Murphy CJ (2001) Seeding growth for size control of 5 – 40 nm Diameter Gold nanoparticles. Langmuir 17:6782–6786. https://doi.org/10.1021/la0104323
Mohamed MB, Ismail KZ, Link S, El-Sayed MA (1998) Thermal reshaping of Gold Nanorods in Micelles. J Phys Chem B 102:9370–9374. https://doi.org/10.1021/jp9831482
Esumi K, Matsuhisa K, Torigoe K (1995) Preparation of Rodlike Gold particles by UV irradiation using Cationic Micelles as a template. Langmuir 11:3285–3287. https://doi.org/10.1021/la00009a002
Sylvestre J-P, Kabashin AV, Sacher E et al (2004) Stabilization and Size Control of Gold Nanoparticles during laser ablation in Aqueous Cyclodextrins. J Am Chem Soc 126:7176–7177. https://doi.org/10.1021/ja048678s
Turkevich J, Stevenson PC, Hillier J (1953) The formation of Colloidal Gold. J Phys Chem 57:670–673. https://doi.org/10.1021/j150508a015
FRENS G (1973) Controlled nucleation for the regulation of the particle size in Monodisperse Gold suspensions. Nat Phys Sci 241:20–22. https://doi.org/10.1038/physci241020a0
Grabar KC, Freeman RGriffith, Hommer MB, Natan MJ (1995) Preparation and characterization of au Colloid Monolayers. Anal Chem 67:735–743. https://doi.org/10.1021/ac00100a008
Brust M, Walker M, Bethell D et al (1994) Synthesis of thiol-derivatised gold nanoparticles in a two-phase liquid–liquid system. J Chem Soc Chem Commun 0:801–802. https://doi.org/10.1039/C39940000801
Brust M, Fink J, Bethell D et al (1995) Synthesis and reactions of functionalised gold nanoparticles. J Chem Soc Chem Commun 1655. https://doi.org/10.1039/c39950001655
Schmid G (1992) Large clusters and colloids. Metals in the embryonic state. Chem Rev 92:1709–1727. https://doi.org/10.1021/cr00016a002
Giersig M, Mulvaney P (1993) Preparation of ordered colloid monolayers by electrophoretic deposition. Langmuir 9:3408–3413. https://doi.org/10.1021/la00036a014
Sardar R, Funston AM, Mulvaney P, Murray RW (2009) Gold nanoparticles: past, Present, and Future. Langmuir 25:13840–13851. https://doi.org/10.1021/la9019475
Daniel M-C, Astruc D (2004) Gold nanoparticles: assembly, supramolecular chemistry, quantum-size-related properties, and applications toward biology, catalysis, and nanotechnology. Chem Rev 104:293–346. https://doi.org/10.1021/cr030698+
Teranishi T, Kiyokawa I, Miyake M (1998) Synthesis of Monodisperse Gold nanoparticles using Linear polymers as Protective agents. Adv Mater 10:596–599. https://doi.org/10.1002/(SICI)1521-4095(199805)10:8%3C596::AID-ADMA596%3E3.0.CO;2-Y
Liu Y, Li N, Zhang Y, Wang Y (2023) Diphenyl imidazole-based fluorescent chemosensor for Al3+ and its Al3+ complex toward water detection in food products. Food Chem 420:136138. https://doi.org/10.1016/j.foodchem.2023.136138
Li C-Y, Zhou Y, Li Y-F et al (2013) Efficient FRET-based colorimetric and ratiometric fluorescent chemosensor for Al3 + in living cells. Sens Actuators B Chem 186:360–366. https://doi.org/10.1016/j.snb.2013.06.039
Hu Q, Liu Y, Li Z et al (2014) A new rhodamine-based dual chemosensor for Al3+ and Cu2+. Tetrahedron Lett 55:4912–4916. https://doi.org/10.1016/j.tetlet.2014.07.040
Zhu G, Huang Y, Wang C et al (2019) A novel coumarin-based fluorescence chemosensor for Al3+ and its application in cell imaging. Spectrochim Acta Mol Biomol Spectrosc 210:105–110. https://doi.org/10.1016/j.saa.2018.11.006
Hossain SM, Singh K, Lakma A et al (2017) A schiff base ligand of coumarin derivative as an ICT-Based fluorescence chemosensor for Al 3+. Sens Actuators B Chem 239:1109–1117. https://doi.org/10.1016/j.snb.2016.08.093
Liu Y, Wu M, Zhao J et al (2022) A simple salicylaldehyde-bearing pyrazine as a turn-on fluorescent chemosensor for Al 3+ and zn 2+ recognition and its applications. Rev Anal Chem 41:217–227. https://doi.org/10.1515/revac-2022-0043
Bai L, Tao F, Li L et al (2019) A simple turn-on fluorescent chemosensor based on Schiff base-terminated water-soluble polymer for selective detection of Al3+ in 100% aqueous solution. Spectrochim Acta Mol Biomol Spectrosc 214:436–444. https://doi.org/10.1016/j.saa.2019.02.062
García-Gutiérrez YS, Huerta-Aguilar CA, Thangarasu P, Vázquez-Ramos JM (2017) Ciprofloxacin as chemosensor for simultaneous recognition of Al3+ and Cu2+ by Logic Gates supported fluorescence: application to bio-imaging for living cells. Sens Actuators B Chem 248:447–459. https://doi.org/10.1016/j.snb.2017.03.140
Kashyap KS, Hira SK, Dey S (2019) Photo-physical aspects of BODIPY-coumarin conjugated sensor and detection of Al 3+ in MCF-7 cell. Supramol Chem 31:695–702. https://doi.org/10.1080/10610278.2019.1659267
Huerta-Aguilar CA, Pandiyan T, Singh N, Jayanthi N (2015) Three novel input logic gates supported by fluorescence studies: Organic nanoparticles (ONPs) as chemo-sensor for detection of Zn2+ and Al3+ in aqueous medium. Spectrochim Acta Mol Biomol Spectrosc 146:142–150. https://doi.org/10.1016/j.saa.2015.03.082
Chatterjee N, Maity SB, Samadder A et al (2016) A chemosensor for Al 3+ ions in aqueous ethanol media: photophysical and live cell imaging studies. RSC Adv 6:17995–18001. https://doi.org/10.1039/C5RA23968K
Bhardwaj V, Bhardwaj K, Sahoo SK (2023) AIE + ESIPT’ active 2-hydroxy-naphthalene hydrazone for the fluorescence turn-on sensing of Al3+. J Fluoresc 33:1157–1164. https://doi.org/10.1007/s10895-022-03138-3
Song I, Torawane P, Lee J-S et al (2021) The detection of Al 3+ and Cu 2+ ions using isonicotinohydrazide-based chemosensors and their application to live-cell imaging. Mater Adv 2:6306–6314. https://doi.org/10.1039/D1MA00564B
Kaur K, Bhardwaj VK, Kaur N, Singh N (2012) Imine linked fluorescent chemosensor for Al3+ and resultant complex as a chemosensor for HSO4 – anion. Inorg Chem Commun 18:79–82. https://doi.org/10.1016/j.inoche.2012.01.018
Tamil Selvan G, Samson J, Rajasingh P et al (2024) A captivating approach to elevate the detection of Al3+ ions incorporates the utilization of a tripodal receptor intricately embellishing the surface of zinc oxide. Spectrochim Acta Mol Biomol Spectrosc 316:124339. https://doi.org/10.1016/j.saa.2024.124339
Kaur K, Chaudhary S, Singh S, Mehta SK (2015) Imine modified ZnO nanoparticles: a luminescent chemodosimeter for Al 3+ and S 2– ions based on ligand displacement reaction. New J Chem 39:1773–1782. https://doi.org/10.1039/C4NJ01372G
Yang Y, Zou T, Zhao R et al (2021) Fluorescence ‘turn-on’ probe for Al 3+ detection in water based on ZnS/ZnO quantum dots with excellent selectivity and stability. Nanotechnology 32:375001. https://doi.org/10.1088/1361-6528/ac0935
Bazany-Rodríguez IvánJ, García‐Rojas LM, Guadalupe Hernández J, Thangarasu P (2024) Sequential Recognition of Bisulfate and Acetate by a Ruthenium(II) Complex: Experimental and Theoretical Studies. ChemPhotoChem 8. https://doi.org/10.1002/cptc.202300145
Cheng J, Zhang Y, Ma X et al (2013) Colorimetric and fluorescent pH and Cu2+ probes induced by photoisomerization of a maleonitrile-based Salen ligand. Chem Commun 49:11791–11793. https://doi.org/10.1039/C3CC47137C
Fatahi Z, Esfandiari N, Ehtesabi H et al (2019) Physicochemical and cytotoxicity analysis of green synthesis carbon dots for cell imaging. EXCLI J 18:454–466. https://doi.org/10.17179/EXCLI2019-1465
Liu KK, Shan CX, He GH et al (2017) Rewritable painting realized from ambient-sensitive fluorescence of ZnO nanoparticles. Sci Rep 7. https://doi.org/10.1038/SREP42232
Singh N, Hundal MS, Hundal G, Martinez-Ripoll M (2005) Zinc templated synthesis—a route to get metal ion free tripodal ligands and lariat coronands, containing Schiff bases. Tetrahedron 61:7796–7806. https://doi.org/10.1016/J.TET.2005.05.052
Martínez-Zepeda DL, Meza-González B, Álvarez-Hernández ML et al (2021) Efficient naked eye sensing of tartrate/malate based on a Zn-Xylenol orange complex in water and membrane-based test strips. Dyes Pigm 188:109239. https://doi.org/10.1016/j.dyepig.2021.109239
Dorazco-González A, Yatsimirsky AK (2010) Binding of ureas and amides to a Cu(II) terpyridine complex in methanol. Inorganica Chim Acta 363:270–274. https://doi.org/10.1016/j.ica.2009.09.019
Afrin A, Jayaraj A, Gayathri MS, P. CAS (2023) An overview of Schiff base-based fluorescent turn-on probes: a potential candidate for tracking live cell imaging of biologically active metal ions. Sens Diagnostics 2:988–1076. https://doi.org/10.1039/D3SD00110E
Carrillo-Carrión C, Nazarenus M, Paradinas SS et al (2014) Metal ions in the context of nanoparticles toward biological applications. Curr Opin Chem Eng 4:88–96. https://doi.org/10.1016/j.coche.2013.11.006
Caballero-Díaz E, Pfeiffer C, Kastl L et al (2013) The toxicity of silver nanoparticles depends on their uptake by cells and thus on their Surface Chemistry. Part Part Syst Charact 30:1079–1085. https://doi.org/10.1002/ppsc.201300215
Wyszogrodzka G, Marszałek B, Gil B, Dorożyński P (2016) Metal-organic frameworks: mechanisms of antibacterial action and potential applications. Drug Discov Today 21:1009–1018. https://doi.org/10.1016/j.drudis.2016.04.009
Martins AF, Follmann HDM, Monteiro JP et al (2015) Polyelectrolyte complex containing silver nanoparticles with antitumor property on Caco-2 colon cancer cells. Int J Biol Macromol 79:748–755. https://doi.org/10.1016/j.ijbiomac.2015.05.036
Guo D, Zhang J, Huang Z et al (2015) Colloidal silver nanoparticles improve anti-leukemic drug efficacy via amplification of oxidative stress. Colloids Surf B Biointerfaces 126:198–203. https://doi.org/10.1016/j.colsurfb.2014.12.023
Sharma S, Chockalingam S, Sanpui P et al (2014) Silver nanoparticles impregnated Alginate–Chitosan-blended Nanocarrier induces apoptosis in human glioblastoma cells. Adv Healthc Mater 3:106–114. https://doi.org/10.1002/adhm.201300090
Yoo HJ, Yoon TH (2014) Flow Cytometric Assessment of reactive oxygen species generations that are directly related to Cellular ZnO Nanoparticle Uptake. J Nanosci Nanotechnol 14:5395–5401. https://doi.org/10.1166/jnn.2014.8733
Song W, Zhang J, Guo J et al (2010) Role of the dissolved zinc ion and reactive oxygen species in cytotoxicity of ZnO nanoparticles. Toxicol Lett 199:389–397. https://doi.org/10.1016/j.toxlet.2010.10.003
Chandrasekaran M, Pandurangan M (2016) In Vitro Selective Anti-proliferative Effect of Zinc Oxide nanoparticles against co-cultured C2C12 Myoblastoma Cancer and 3T3-L1 normal cells. Biol Trace Elem Res 172:148–154. https://doi.org/10.1007/s12011-015-0562-6
Yu MO, Song N-H, Park K-J et al (2015) Romo1 is associated with ROS production and cellular growth in human gliomas. J Neurooncol 121:73–81. https://doi.org/10.1007/s11060-014-1608-x
Liou G-Y, Storz P (2010) Reactive oxygen species in cancer. Free Radic Res 44:479–496. https://doi.org/10.3109/10715761003667554
Couvreur P, Vauthier C (2006) Nanotechnology: Intelligent Design to treat Complex Disease. Pharm Res 23:1417–1450. https://doi.org/10.1007/s11095-006-0284-8
Crommelin DJA, Florence AT (2013) Towards more effective advanced drug delivery systems1. Int J Pharm 454:496–511. https://doi.org/10.1016/j.ijpharm.2013.02.020
Acknowledgements
The authors thank Ivan Puente Lee at USAI, UNAM for TEM analyses. Iván Jonathan Bazany Rodríguez is grateful to CONAHCYT, Mexico for his post-doctoral scholarship (CVU: 662748). We acknowledge OPEX EIC- PHFT070-23ES00007-04-001 at Tecnológico de Monterrey, Puebla, and ITESM UOttawa Seed Grant Program 23-25 (Project No. IJST070-23OT77001-UO-TEC Revalorization of steel residues) as well as Dirección General de Asuntos de Personal Académico (Project PAPIIT No IN202622).
Funding
Dirección General de Asuntos de Personal Académico (Project PAPIIT No IN202622).
Author information
Authors and Affiliations
Contributions
Carlos Alberto Huerta-Aguilar: ZnO NPs preparation and characterization; Iván J. Bazany-Rodríguez: sensing and recognition studies; Valeria Hansberg-Pastor: cell toxicity analysis; Ignacio Camacho-Arroyo: fluorescence glioblastoma U251 image studies; Iván Alejandro Reyes-Dominguez: Transmission electron microscopy (TEM) studies; Pabel Antonio Cervantes-Avilés: Dynamic Light Scattering studies and analysis; Pandiyan Thangarasu, XRD analysis and all analyses of results, and revision of manuscript.
Corresponding author
Ethics declarations
Ethics Approval
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Huerta-Aguilar, C.A., Bazany-Rodríguez, I.J., Hansberg-Pastor, V. et al. ZnO-Salen NPs Employed as Chemosensor for Detection of Al3+ and K+ in Aqueous Medium, Developing Human Cell Images. J Fluoresc (2024). https://doi.org/10.1007/s10895-024-03913-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10895-024-03913-4