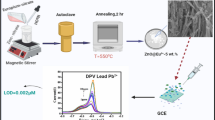
Abstract
Solvatochromic studies in conjunction with NCQDs and analysis of material at different pH levels provide valuable insights about the process of metal ion sensing. Metal ion sensing holds significant importance in various fields like environment monitoring, biomedical diagnostics and various industrial purpose. The detection of metal ions by mixing the nitrogen-doped quantum dots (NCQDs) in the solvent at different pH levels for the analysis of the photoluminescence spectra is the unique property to achieve selective metal ion detection. In present study, the synthesis of NCQDs was performed by the use of flowers of Tecoma stans. The synthesis of NCQDs to best of our knowledge using flowers of Tecoma stans as natural carbon source via hydrothermal process has been done for the first time. The NCQDs exhibit absorption bands ranging from 190 to 450 nm, with the energy band gap varying from 3.55 to 5.42 eV when mixed with different solvent such as, 1–4 dioxane, acetone, acetonitrile, ethyl- acetate, ethanol, methanol and toluene. The fluorescence spectra exhibited highly intense range from approximately 390 to 680 nm across various solvents. XRD analysis further confirmed the crystalline nature of the particles with an average size of 6.96 nm. Different peak positions of the FTIR spectra support functional groups having C-H stretching, C = O (carbonyl) stretching, and C = C stretching vibrations. In the study a notable solvatochromic shift was observed, indicating sensitivity to change in solvent polarity. Additionally, the investigation of the ratio of ground to excited state dipole moment based on solvatochromic shift yielded a value of 3.30. This provide valuable information about optical and electronic properties of NCQDs. Overall, our study sheds light on the unique properties of NCQDs synthesized from Tecoma stans flowers and their potential applications in metal ion sensing, pH probing, and solvent polarity studies.








Similar content being viewed by others
Data Availability
No datasets were generated or analysed during the current study.
References
Sharma VD, Vishal V, Chandan G, Bhatia A, Chakrabarti S, Bera MK (2022) Green, sustainable, and economical synthesis of fluorescent nitrogen-doped carbon quantum dots for applications in optical displays and light-emitting diodes. Mater Today Sustain 19:100184. https://doi.org/10.1016/j.mtsust.2022.100184
Dhariwal J, Rao GK, Vaya D (2024) Recent advancements towards the green synthesis of carbon quantum dots as an innovative and eco-friendly solution for metal ion sensing and monitoring. RSC Sustain 2:11–36
Badilla AN, Ayala GC, Beleño YD, Sánchez MCH, Hurtado RB, Pérez JEL, Macias AH, Valadez MC (2023) Green Synthesis for Carbon Quantum Dots via Opuntia ficus-indica and Agave maximiliana: surface-enhanced Raman scattering sensing applications, ACS omega. 8(37):33342–33348. https://doi.org/10.1021/acsomega.3c02735
Sharma A, Das J (2019) Small molecules derived carbon dots: synthesis and applications in sensing, catalysis, imaging, and biomedicine. J Nanobiotechnol 17:92. https://doi.org/10.1186/s12951-019-0525-8
Yang X, Wang D, Luo N, Feng M, Peng X, Liao X (2020) Green synthesis of fluorescent N,S-carbon dots from bamboo leaf and the interaction with nitrophenol compounds. Spectrochim Acta Part A Mol Biomol Spectrosc 239:118462. https://doi.org/10.1016/j.saa.2020.118462
Ozyurt D, Al. M, Kobaisi RK, Hocking B, Fox (2023) Properties, synthesis, and applications of carbon dots: a review. Carbon Trends 12:100276. https://doi.org/10.1016/j.cartre.2023.100276
Zhou C, Du J, Zhao H, Xiong Z, Zhao L (2023) Green synthetic carbon quantum dots based on waste tobacco leaves and its application to detecting borax content in flour and its products. J Mol Struct 1278:134959. https://doi.org/10.1016/j.molstruc.2023.134959
Devi P, Saini S, Kim K-H (2019) The advanced role of carbon quantum dots in nanomedical applications. Biosens Bioelectron 141:111158. https://doi.org/10.1016/j.bios.2019.02.059
Liu H, Li Y, Sun K, Fan J, Zhang P, Meng H, Yang B (2015) Nitrogen-doped carbon quantum dots with oxygen-rich functional groups. J Am Chem Soc 137:5310–5317
Wang Y, Hu A, Carbonaro RF (2019) Enhanced fluorescence emission of nitrogen-doped carbon quantum dots by localized surface plasmon resonance. J Lumin 212:60–64
Wang C, Hu T, Wen Z, Zhou J, Wang X, Wu Q (2018) Concentration-dependent color tunability of nitrogen-doped carbon dots and their application for iron (III) detection and multicolor bioimaging. J Colloid Interface Sci 521:33–41. https://doi.org/10.1016/j.jcis.2018.03.021
Zhang Y, Wang Y, Feng X, Zhang F, Yang Y (2016) Effect of reaction temperature on structure and fluorescence properties of nitrogen-doped carbon dots. Appl Surf Sci 387:1236–1246. https://doi.org/10.1016/j.apsusc.2016.07.048
Esmaeili M, Wu Z, Chen D, Singh A, Sonar P (2022) Composition and concentration-dependent photoluminescence of nitrogen-doped carbon dots. Adv Powder Technol 33:103560. https://doi.org/10.1016/j.apt.2022.103560
Kaur J, Sharma S, Mehta SK, Kansal SK (2020) Highly photoluminescent and pH sensitive nitrogen doped carbon dots (NCDs) as a fluorescent sensor for the efficient detection of cr (VI) ions in aqueous media. Spectrochim Acta Part A Mol Biomol Spectrosc 227:117572. https://doi.org/10.1016/j.saa.2019.117572
Moniruzzaman M, Kim J (2019) N-doped carbon dots with tunable emission for multifaceted application: solvatochromism, moisture sensing, pH sensing, and solid state multicolor lighting. Sens Actuators B 295:12–21. https://doi.org/10.1016/j.snb.2019.05.035
Li H, Kang Z, Liu Y, Lee ST (2012) Carbon nanodots: synthesis, properties and applications. J Mater Chem 22:24230–24253. https://doi.org/10.1039/C2JM34690G
Wang F, Chen YH, Liu CY, Ma D (2013) White-light-emitting CdSe quantum dots/ nitrogen-doped carbon dots: toward application in white light-emitting diodes. Angew Chem Int Ed 52:1–5
Li YX, Lee JY, Hu HLCC, Chiu TC (2021) Highly fluorescent nitrogen-doped carbon dots for selective and sensitive detection of Hg2+ and ClO– ions and fluorescent ink. J Photochem Photobiol A 405:112931. https://doi.org/10.1016/j.jphotochem.2020.112931
Echeverry-Gonzalez CA, Kouznetsov VV (2021) Carbon Dots: An Insight into Their Application in Heavy Metal Sensing Recent Progress in Materials. Recent Progress Mater. https://doi.org/10.21926/rpm.2102015
Huang SW, Lin YF, Li YX, Hu CC, Chiu TC (2019) Synthesis of fluorescent Carbon dots as selective and sensitive probes for cupric ions and cell imaging. Molecules 24:1785. https://doi.org/10.3390/molecules24091785
Tangod VB, Mastiholi BM, Raikar P, Kulkarni SG, Raikar US (2015) Studies of the photophysics of highly fluorescent red mega 480 laser dye in solutions: steady state spectroscopy. Spectrochim Acta Part A Mol Biomol Spectrosc 148:105–113. https://doi.org/10.1016/j.saa.2015.03.119
Chemla DS, Zyss J (1987) Non-linear Optical Properties of Organic Molecules and Crystals, Academic Press, 1, New York. https://doi.org/10.1016/B978-0-12-170611-1.X5001-3
Liptay W (1974) Dipole Moments and Polarizabilities of Molecules in Excited Electronic States, Excited states, 1, Academic Press, NewYork. https://doi.org/10.1016/B978-0-12-227201-1.50009-7
Kawski A, Rabek JF (1992) Progress in Photochemistry and Photophysics, fifth edn. CRC, Boca Raton
Lombardi JR (1970) Correlation between structure and dipole moments in the excited states of substituted benzenes. J Am Chem Soc 92:1831–1833
De Haas MP, Warman JM (1982) Photon-induced molecular charge separation studied by nanosecond time-resolved microwave conductivity. Chem Phys 73:35–53. https://doi.org/10.1016/0301-0104(82)85148-3
Mohammad-Jafarieh P, Akbarzadeh A, Salamat R, Jamshidi-Ghaleh K (2021) Solvent effect on the absorption and emission spectra of carbon dots: evaluation of ground and excited state dipole moment. BMC Chem 15:53. https://doi.org/10.1186/s13065-021-00779-6
Zakerhamidi MS, Ghanadzadeh A, Moghadam M (2012) Solvent effects on the UV/Visible absorption Spectra of some Aminoazobenzene Dyes. Chem Sci Trans 1:1–8. https://doi.org/10.7598/cst2012.118
Koutek B (1978) Dipole moments in excited state. Statistical investigation of methods employing solvatochromism. Collect Czechoslov Chem Commun 43:2368–2386. https://doi.org/10.1135/cccc19782368
Woldegiorges K, Belay A, Kebede A, Abebe T (2021) Estimating the ground and excited state dipole moments of levofloxacin and norfloxacin drugs using Solvatochromic effects and computational work. J Spectrosc. https://doi.org/10.1155/2021/7214182
Pandey N, Tewari N, Pant S, Mehata MS (2022) Solvatochromism and estimation of ground and excited state dipole moments of 6-aminoquinoline. Spectrochim Acta A Mol Biomol Spectrosc 267:120498. https://doi.org/10.1016/j.saa.2021.120498
Saroj MK, Sharma N, Rastogi RC (2012) Photophysical Study of some 3 Benzoylmethyleneindol-2-ones and estimation of Ground and Excited States Dipole moments from Solvatochromic Method using Solvent Polarity parameters. J Mol Struct 1012:73–86. https://doi.org/10.1016/j.molstruc.2011.12.043
Joshi S, Bhattacharjee R, Varma YT, Pant DD (2013) Estimation of ground and excited state dipole moments of quinidine and quinidine dication: experimental and numerical methods. J Mol Liq 179:88–93. https://doi.org/10.1016/j.molliq.2012.11.023
Joshi S, Pant DD (2012) Ground and excited state dipole moments of quinine sulfate dication: solvatochromic shift of absorption and fluorescence spectra. J Mol Liq 172:125–129. https://doi.org/10.1016/j.molliq.2012.04.002
Mangalagiu II, Florescu M, Zbancioc G, Caprosu M, Solvatochromic Study of Two Pyridazinium Ylids Binary, Mangalagiu II, Florescu M, Zbancioc G, Caprosu M (2007) Solvatochromic Study of Two Pyridazinium Ylids Binary Solutions. J Phys Conf Ser 61:484–486. https://doi.org/10.1515/kbo-2016-0103
Zbancioc G, Huhn T, Groth U, Deleanu C, Mangalagiu II (2010) Pyrrolodiazine derivatives as blue organic luminophores: synthesis and properties. Part 3. Tetrahedron. 66 (2010) 4298–4306 66:4298–4306. https://doi.org/10.1016/j.tet2010.04.050
Singh S, Miller CT, Singh P (2024) A comprehensive review on ecology, life cycle and use of Tecoma stans (bignoneaceae). Bot Stud. https://doi.org/10.1186/s40529-024-00412-4
Sbihi HM, Mokbli S, Nehdi IA, Al-Resayes SI (2015) Physico-chemical properties of Tecoma stans. Linn. Seed oil: a new crop for vegetable oil. Nat Prod Res 29:1249–1255. https://doi.org/10.1080/14786419.2015.1024118
Taher MA, Dawood DH, Sanad MI, Hassan RA (2016) Searching for anti-hyper glyce micphyto molecules of Tecoma stans. Eur J Chem 7:397–404. https://doi.org/10.5155/eurjchem.7.4.397-404.1478
Bakr RO, Fayed MA, Salem MA, Hussein AS (2019) Tecoma stans: alkaloid profile and antimicrobial activity. J Pharm Bioallied Sci 11:341–347
Khatak S, Mali DK, Dahiya R (2019) Tecoma stans: a noxious weed put to beneficial use. Int J Chem Stud 7:296–299
Gan YX, Jayatissa AH, Yu Z, Chen X, Li M (2020) Hydrothermal synthesis of nanomaterials. J Nanomater. https://doi.org/10.1155/2020/8917013
Tauc J, Grigorovici R, Vancu A (1966) Optical properties and electronic structure of amorphous germanium. Phys Status Solidi (b) 15:627–637. https://doi.org/10.1002/pssb.19660150224
Bouafia A, Laouini SE, Tedjani ML, Ali GAM, Barhoum A (2022) Green biosynthesis and physicochemical characterization of Fe3O4 nanoparticles using Punica granatum L. fruit peel extract for optoelectronic applications. Text Res J 92:2685–2696
Gherbi B, Laouini SE, Meneceur S, Bouafia A, Hemmami H, Tedjani ML, Thiripuranathar G, Barhoum A, Menaa F (2022) Effect of pH value on the bandgap energy and particles size for biosynthesis of ZnO nanoparticles: efficiency for photocatalytic adsorption of methyl orange. Sustainability 14:11300. https://doi.org/10.3390/su141811300
Joshi NC, Kumar N (2022) Potential of PTH-Fe3O4 based nanomaterial for the removal of pb(II),cd(II),andCr(VI)ions. J Inorg Organomet Polym Mater 32:1234–1245. https://doi.org/10.1007/s10904-021-02173-0
Nguyen TN, Le PA, Phung VBT (2020) Facile green synthesis of carbon quantum dots and biomass-derived activated carbon from banana peels: synthesis and investigation. Biomass Convers Biorefinery. https://doi.org/10.1007/s13399-020-00839-2
Li Y, Zhang Q, Zhang J, Jin L, Zhao X, Xu T (2015) A top-down approach for fabricating free-standing bio-carbon supercapacitor electrodes with a hierarchical structure. Sci Rep 5:14155
Tsade H, Abebe B, Murthy HCA (2019) Nano sized Fe–Al oxide mixed with natural maize cob sorbent for lead remediation. Mater Res Express 6. https://doi.org/10.1088/2053-1591/ab1a80
Perumal S, Atchudan R, Thirukumaran P, Yoon DH, Lee YR, Cheong IW (2022) Simultaneous removal of heavy metal ions using carbon dots-doped hydrogel particles. Chemosphere 286. https://doi.org/10.1016/j.chemosphere.2021.131760
Hou Y, Zhang R, Cheng H, Wang Y, Zhang Q, Zhang L, Wang L, Li R, Wu X, Li B (2023) Mg2+doped carbon dots synthesized based on Lycium ruthenicum in cell imaging and promoting osteogenic differentiation in vitro. Colloids Surf Physicochem Eng Asp 656:130264. https://doi.org/10.1016/j.colsurfa.2022.130264
Wu P, Li W, Wu Q, Liu Y, Liu S (2017) Hydrothermal synthesis of nitrogen-doped carbon quantum dots from microcrystalline cellulose for the detection of Fe3 + ions in an acidic environment. RSC Adv 7:44144–44153. https://doi.org/10.1039/C7RA08400E
Shaikh AF, Tamboli MS, Patil RH, Bhan A, Ambekar JD, Kale BB (2019) Bioinspired Carbon Quantum dots: an Antibiofilm Agent. J Nanosci Nanotechnol 19:2339–2345. https://doi.org/10.1166/jnn.2019.16537
Feng Z, Li Z, Zhang X, Shi Y, Zhou N (2017) Nitrogen-Doped Carbon Quantum dots as fluorescent probes for sensitive and selective detection of Nitrite. Molecules 22:2061. https://doi.org/10.3390/molecules22122061
Wang ZL (2001) Transmission electron microscopy and spectroscopy of nanoparticles. In: Characterization of Nanophase Materials, 18, Wiley, Weinheim, Germany. https://doi.org/10.1002/1521-4117(200110)18:3%3C142::AID-PPSC142%3E3.0.CO;2-N
Shen L, Zhang L, Chen M, Chen X, Wang J (2013) The production of pH-sensitive photoluminescent carbon nanoparticles by the carbonization of polyethylenimine and their use for bioimaging. Carbon 55:343–349. https://doi.org/10.1016/j.carbon.2012.12.074
Nie H, Li M, Li Q, Liang S, Tan Y, Sheng L, Shi W, Zhang SX (2014) Carbon dots with continuously tunable full-Color Emission and their application in Ratiometric pH sensing. Chem Mater 26:3104–3112. https://doi.org/10.1021/cm5003669
Tan XW, Romainor ANB, Chin SF, Ng SM (2014) Carbon dots production via pyrolysis of sago waste as potential probe for metal ions sensing. J Anal Appl Pyrol 105:157–165. http://hdl.handle.net/1959.3/371621
Pu J, Liu C, Wang B, Liu P, Jin Y, Chen J (2021) Orange red-emitting carbon dots for enhanced colorimetric detection of Fe3+. Analyst 146:1032–1039. https://doi.org/10.1039/D0AN02075C
Kumari R, Varghese A, George L, Sudhakar SYN (2017) Effect of solvent polarity on the photophysical properties of chalcone derivatives. RSC Adv 7:24204–24214. https://doi.org/10.1039/C7RA01705G
Parkanyi C, Aaron JJ (1998) Dipole moments of aromatic heterocycles. Theor Comput Chem 5:233–258. https://doi.org/10.1016/S1380-7323(98)80011-3
Adenier A, Aaron JJ, Parkanyi C, Deng G (1996) Sallah, solvent effects on the electronic absorption and fluorescence emission spectra of merocyanine 540-a biological probe. Heterocycl Commun 2:403–408. https://doi.org/10.1515/HC.1996.2.5.403
Acknowledgements
Dr. B.S. Rawat, one of the contributors, expresses gratitude to the Division of research & Innovation, Uttaranchal University, Dehradun for facilitating the research facilities.
Funding
The present work was carried out with the support of seed money (Ref: UU/DRI/SM/2023-24/008) provided by Uttaranchal University, Dehradun.
Author information
Authors and Affiliations
Contributions
Dr. Poonam Negi supervised the article and conducted the characterization part, Dr. Bhupendra Singh Rawat conceptualized and performed the analysis of the dipole moment, Dr. Naveen Chandra Joshi was responsible for the experimental work on heavy metal detection and Dr. Narinder Kumar was for the interpretation of the same, Dr. Kanak Pal Singh Parmar and Dr. Shuchi Upadhyay focused on the experiments and analysis of the fluorescence part, Dr. Vinod Singh curated the data.
Corresponding author
Ethics declarations
Ethical Approval
Not applicable.
Consent to Participate
All the authors consent to publishing the paper.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Negi, P., Rawat, B.S., Joshi, N.C. et al. Green Synthesis of Highly Fluorescent NCQDs: A Comprehensive Study on Synthesis, Characterization, Photophysical Properties, pH Sensing, Heavy Metal Detection, and Solvatochromic Behavior through Hydrothermal Method. J Fluoresc (2024). https://doi.org/10.1007/s10895-024-03710-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10895-024-03710-z