Abstract
Olive oils are more expensive compared with other vegetable oils. Therefore, adulterating such expensive oil is prevalent. The traditional methods for olive oil adulteration detection are complex and require pre-analysis sample preparation. Therefore, simple and precise alternative techniques are required. In the present study, the Laser-induced fluorescence (LIF) technique was implemented for detecting alteration and adulteration of olive oil mixed with sunflower or corn oil based on the post-heating emission characteristics. Diode-pumped solid-state laser (DPSS, λ = 405 nm) was employed for excitation and the fluorescence emission was detected via an optical fiber connected to a compact spectrometer. The obtained results revealed alterations in the recorded chlorophyll peak intensity due to olive oil heating and adulteration. The correlation of the experimental measurements was evaluated via partial least-squares regression (PLSR) with an R-squared value of 0.95. Moreover, the system performance was evaluated using receiver operating characteristics (ROC) with a maximum sensitivity of 93%.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Olive oil is one of the vegetable oils which are very valued for the health of different organs especially the brain, the heart and the nervous system. It is rich in monounsaturated fatty acids, bioactive compositions, antioxidants, vitamins (K and E), and minerals [1]. Additionally, it needs minimum processing procedures for removing impurities and retaining its natural flavor. It is mechanically obtained from olive tree fruits and exposed to some treatment processes including washing, decantation, centrifugation, and filtration [2]. Commercially, the price of different types of olive oils is relatively higher than other vegetable oils such as soybean or sunflower oils. Therefore, adulteration of olive oil with these lower-priced oils is common and precise and fast techniques for detecting adulteration are encouraged. Currently, some chemical analyses based on gas-liquid chromatography are applied for the identification and quantification of adulterants in olive oils [3], however, most of the common chemical techniques are time consuming, costly, and require complex sample preparation in addition to the use of toxic chemicals [4, 5]. Alternatively, some analytical techniques including vibrational, chromatographic and thermal techniques can be utilized [6]. Moreover, spectroscopic techniques such as laser-induced breakdown spectroscopy [7, 8], Raman spectroscopy [9] and near-infrared spectroscopy [10] have been extensively utilized in the inspection of the quality and possible adulteration in different food products including olive oil [11,12,13].
Laser-induced fluorescence (LIF) spectroscopy is a very promising non-invasive optical modality utilized in various medical and industrial applications [14]. It has been applied for biological investigations [15, 16], tumor classification [17] and food adulteration detection [18] and quality inspection [8]. Regarding olive oil adulteration detection, LIF combined with different chemometric techniques has been widely employed to examine extra virgin olive oil blended with different vegetable oils [19]. It has been also utilized to monitor virgin olive oils at different storage conditions [20] and assess their quality [21, 22]. Nikolova et al. [23] analyzed the fluorescence spectra of olive oil adulterated with sunflower oil at different excitation wavelengths based on the tocopherol and fatty acid content using fiber optics spectrometer. Additionally, Mu et al. [24] proposed a portable system to detect and quantify extra virgin olive oil (EVOO) adulterated with rapeseed, peanut, and blend oils using 473-nm LIF. Recently, Zhang et al. [25] employed ultra-violet to blue LED-induced fluorescence spectroscopy to quantitatively detect adulteration in extra-virgin olive oil mixed with peanut oil, and soybean oil. Moreover, Abedin [26] studied the LIF spectra of various edible oils such as mustard, sunflower, corn, sesame, peanut, rice bran, flaxseed and olive to investigate their molecular features. LIF spectroscopic technique is preferred because it is safe, portable, needs almost no sample preparation and has relatively fast spectral acquisition. However, the conventional LIF scheme may encounter some noise that can overshadow its sensitivity and specificity [27].
The recently published results reveal that the storage period and freshness of olive oils affects its quality and hence affects the intensity of the fluorescence emission [28, 29]. For cooking purposes, olive oil could be heated above its smoke temperature [30]. Upon heating, some chemical reactions occurs including oxidation and polymerization which affect the olive oil nutritional compounds [31]. The thermal oxidation characteristics were utilized in quantifying the low concentration of adulterants (2% soybean oil) in extra-virgin olive based on LIF spectroscopy combined with chemometric analysis showing enhanced LIF spectra of the heated oil samples [32]. The present study has double aims; the first aim is to investigate the effect of heating on the florescence characteristics of EVOO. While the second objective is to employ the post-heating fluorescence modifications to detect adulteration in EVOO with sunflower and corn oils. To this end, the oil samples (pure and adulterated) were heated to smoke temperature (190 °C) and the resultant LIF spectra have been recorded and analyzed. The implemented method has been evaluated using receiver operating characteristic curve (ROC) and the experimental measurements have been statistically verified using partial least squares regression (PLSR) method.
Methods
Laser-induced Fluorescence (LIF)
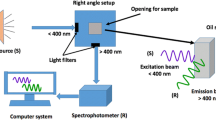
The fluorescence measurements of different oil samples have been performed based on the optical configuration presented in Fig. 1. Due to the various fluorescence properties of olive oil components, different wavelengths could be used for excitation (typically from 270 to 458 nm) [33]. However, the 405-nm irradiation is commonly employed for obtaining the fluorescence emission of chlorophylls [26, 34, 35]. Therefore, a continuous-wave diode pumped solid-state (DPSS) laser source (XL-R405SD, Xinland international Co., Ltd, China) at wavelength of 405 nm and output power of 100 mW, has been used as the excitation source in the present work. The laser beam diameter is about 2 mm.
Upon interacting with the tested oil samples, the resultant fluorescence emission has been delivered to a digital spectrometer (STDFSM, Touptek Photonics Co. Ltd, Zhejiang China) via an optical fiber (200 µm core diameter and SMA 905 interface type). The distance between the laser source and the sample was identical to the distance between the sample and collecting fiber (~ 6.7 cm). The angle between the laser beam and the optical fiber with respect to the sample was 90°. The spectrometer was connected to the laptop computer through a USB cable. The measuring detector (Toshiba TCD1304AP Linear CCD array (Sony ILX511 2048 Linear CCD array)) has a 200-1100 nm response range, 3648 pixels and 8×200 µm pixel size. Data processing and analysis have been performed using the spectrometer software (Toup Spm) and Matlab R2018b.
Sample Selection and Preparation
Virgin olive oil (VOO), EVOO, sunflower oil, and corn oil samples have been purchased from different local hypermarkets. VOO and EVOO were initially investigated and the preliminary results revealed the higher fluorescence emission peak of the EVOO compared to VOO. Therefore, the rest of the experiments were performed using EVOO. To confirm that the EVOO samples purchased were definitely of extra virgin grade, the amount of chlorophyll-a in the tested EVOO (which was = 16.91 mg/kg) was measured using UV-VIS spectroscopy (UV-1750 spectrophotometer, Shimadzu, Kyoto, Japan). When compared to other commercially available brands in Egypt (such as Tiara, Khoshala, El-Salheya, and Wadi food, Avanti-branded EVOO had showed higher chlorophyll-a content as shown in Fig. 2 Therefore, it was chosen for the analysis.
A total 90 samples have been examined which were divided into 30 EVOO samples (brand name: Avanti) and 30 EVOO mixed with sunflower oil (brand name: Hla) and 30 EVOO mixed with corn oil (brand name: Roots), 1 mL each volume. The 30 samples of each oil category were identical products from the same brand that were purchased from several market places and had manufacturing dates that varied from January 2021 to March 2022. All the studied oil types were made in Egypt. The investigated samples were placed in a 24-wells plate using pipette. Sample heating has been achieved via a digital hotplate and a magnetic stirrer (MSH-10, DAIHAN Scientific - Korea). All samples were stored in the dark at room temperature 23 °C during the measurements time. The temperature during experiments was measured and controlled using a digital temperature controller and a thermocouple (XH-W3001, Generic, China).
Partial Least Squares Regression (PLSR)
In the current investigations, LIF spectra of the examined oil samples were validated using the multivariate calibration technique Partial Least Squares Regression (PLSR). PLSR is applied to model response variable of highly correlated large number of predictor variables datasets via creating new predictors called “components” [36]. Unlike principle component regression (PCR) method, PLSR components that demonstrate the observed variability in the predictors have been constructed taking the response variable into account [37, 38]. Generally, the regression is used to determine the best-fit line to the dataset. Hence ensuring that the measured data is correlated and there are no odd values [39]. Based on a Matlab function named “plsregress”, PLSR of the obtained LIF spectra has been implemented in the current study. After defining the number of the PLS components, a partial least-squares regression of the response matrix on the predictor variables matrixes computed, then the predictor and response loadings were returned. Furthermore, R-squared value and root mean square error were estimated. In matrix representation, PLSR method is based on the following relations [40, 41]:
where, N is the principal component, Y is the set of the observed responses, W is the set of the composing weights, P is the set of the principal component loadings and E is the residual variance. The singular value decomposition is then used for matrix solution without any matrix inversions as follows:
where, W is the left singular vectors orthonormal matrix with W×W’ = I (the identity matrix), P is the right singular values orthonormal matrix and D is the singular values diagonal matrix.
Receiver Operator Curve (ROC)
ROC curve is obtained by plotting sensitivity versus “1- specificity” for classification purposes [42, 43]. It evaluates the performance of the applied method according to the acquired area under the ROC curve (AUC) [44, 45]. This curve is created based on “true positive, true negative, false positive, and false negative" concept [46]. In the present work, the ROC curves of the obtained results have been created using MATLAB in-house function. The LIF spectra of olive oil and olive oil mixed with sunflower or corn oils were used as the two classes of data for discrimination. At any point p, the test’s sensitivity (the true positive rate \(TPR=\frac{true\_positive}{true\_positive+false\_negative}\)) is represented by Se(p) = 1 − G(p) and its specificity (the false positive rate \(FPR=\frac{false\_positive}{false\_positive+true\_negative}\)) is given by Sp(p) = F(p). where, F and G are the distribution functions of X and Y which are two independent random variables denoting the measured data sets, respectively [47]. Then, the ROC curve is constructed by plotting the Se(p) versus 1−Sp(p) for -∞ ≤ p ≤ ∞.
Results and Discussion
LIF Spectra of Different Oil Samples
LIF spectra of olive oils (VOO and EVOO) were investigated. The obtained Florescence spectra of 30 samples of EVOO and VOO samples were averaged and presented in Fig. 3.
The 675-nm emission peak of VOO is almost 33% lower than EVOO. Although, VOO and EVOO are made from olives, they are extracted via different methods. Therefore, they have different colors, tastes and nutritional benefits [48]. Moreover, relevant literature revealed that EVOO contains higher polyphenols and chlorophylls (and their derivatives) contents than VOO [49, 50] which may explain the lower 675-nm emission peak in VOO. Accordingly, experiments were performed using EVOO. Upon excitation with the incident laser light (405 nm), EVOO showed an emission peak at 675-nm which was associated with the presence of chlorophyll [51]. The fluorescence emission of EVOO in comparison with that of the corn and sunflower oils is illustrated in Fig. 4a. Additionally, variations in the recorded chlorophyll peak of unadulterated and adulterated EVOO are shown in Fig. 4b.
As demonstrated in Fig. 3a, the sunflower and corn oils have no fluorescence peaks. While in adulterated EVOO about 30% and 34% decrease in the overall fluorescence spectra has been observed for olive oil mixed with sunflower oil or corn oil respectively as presented in Fig. 4b.
Although it is documented in the literature that fluorescence emission of olive oil can be possible below 600 nm [23, 26], in the present investigations we only considered the change in the chlorophyll peak which is considered the highest recorded fluorescence peak (λ=675-nm) in olive oils [52]. Therefore, we specified the bandwidth to be from 600 – 800 nm.
Post-heating LIF Spectra in Unadulterated and Adulterated Oil Samples
EVOO samples were heated to its smoke point (about 190 °C) and their fluorescence spectra have been recorded and analyzed. As illustrated in Fig. 5a, the peak representing the chlorophyll (λ=675-nm) significantly decreased (about 62%) directly after heating., After 15 minutes the samples were returned to its initial temperate (23 °C) and the chlorophyll peak started to increase (61% recovery from original) however, it didn’t return to its normal value. On the other hand, the chlorophyll peak in EVOO adulterated with corn or sunflower oil attenuated by 30% and 20% respectively after heating to the smoke point as demonstrated in Fig. 5b and c, respectively. In the EVOO adulterated with corn oil, an increase (about 16%) in the fluorescence peak was noticed after the returning to its initial temperature (before heating). However, the fluorescence peak of the EVOO adulterated with sunflower oil remained constant and did not increase as occurred in the unadulterated EVOO or EVOO adulterated with corn oil. All the graphs were obtained utilizing 30 different samples (five replicates each) of the same blend (1:1 blending ratio) using different purchased products of the same brand.
Some physical changes such as viscosity increase or changing in color (darkening) may occur in vegetable oils after heating to a high temperature (i.e. >180 °C). Moreover, significant physical and chemical reactions including hydrolysis, oxidation, cyclization, and polymerization can be also arisen [53]. Such changes influence the sensory, taste, flavor and quality of the oils. Consequently, oil degradation resulting from heating to smoke point (thermal oxidation) may be responsible for the intensity reduction occurred in the recorded fluorescence peaks after heating [54]. Additionally, the repeated heating of EVOO (to the smoke point) revealed a continuous reduction in the chlorophyll peak in the recorded fluorescence emission as illustrated in Fig. 6. During this investigation, the temperature of the sample was recorded before the start of the experiment, and measurements were made after the heating process was complete before the samples were allowed to cool back to their initial temperature. Time interval for the whole process did not exceed 10 minutes.
In comparison with literature, Rasul and Inanc [55] showed that heating VOO to high temperatures (≥150 °C) results in a significant decrease in its chlorophyll content (about 99.5% after heating for 24 hr. at 200 °C). Moreover, Balaky et al. [56] studied the change in chlorophyll contents in addition to the oxidative stability in olive pomace oil due to different-period repetitive-heating procedures. Their results revealed a significant decrease (below 2.7%) in the chlorophyll contents after heating. However, in most of the previous studies, the change in the chlorophyll content was inspected based on analyzing the absorbance of the oil samples at different wavelengths using a spectrophotometer. Nevertheless, Cheikhousman et al. [52] have utilized fluorescence spectroscopy (excitation wavelengths at 330 and 450 nm) to monitor the deterioration occurred in EVOO upon heating [54]. Additionally, Saleem et al. [57] utilized the fluorescence spectroscopy (350-nm excitation source) to study the heating effect (from 140 to 180 °C) on EVOO to inspect its safety for cooking. Their results disclosed a decrease in the overall LIF intensity (including chlorophyll peak) after heating the oil samples above 150 °C, which agrees with our obtained results.
Although the present study aimed to investigate the post-heating chlorophyll fluorescence characteristics of unadulterated and adulterated EVOO, the use of a single excitation wavelength is considered a limitation of the present study. Consequently, further investigations for the post-heating fluorescence emission of EVOO are needed using other excitation wavelengths to analyze other contents rather than chlorophylls.
PLSR of LIF Spectra
The correlation between the obtained LIF spectral measurements were statistically evaluated using the Partial Least Squares regression (PLSR) method. The loaded data in the PLSR model were the spectral intensities of the 30 samples unadulterated and adulterated (with corn or sunflower) EVOO, respectively. The “plsregress” Matlab function was used to fit the PLSR model with three PLS components. It is worthy to mention here that the number of components was specified according to the cross-validation method [58]. The predictive ability of the PLS model was assessed based on the coefficient of determination (R2) between observed data and fitted response values and the root mean square error (RMSE). Figure 7 shows the relation between the observed and predicted values based on our measured spectroscopic data. The calculated R2-value was 0.946 and the RMSE ~ 2.056 which indicate a good model performance for adulteration detection [59].
It is worthy to mention here that, PLSR was not implemented to reflect the detection performance of the proposed method. However, it employed to find the correlation between the obtained repeated spectral measurements. Other technique such as PCA has been widely utilized to statically analyze the adulteration detection models, which is not the aim of our study.
ROC Curve Analysis
The ROC curves for the discrimination between unadulterated and adulterated olive oil (with sunflower or corn oil) are presented in Fig. 8. The obtained ROC curves showed significant characteristics with sensitivity of 93%, 88%, for sunflower-adulterated EVOO and Corn-adulterated EVOO, respectively. The area under the curve (AUC) exceeds 75% in both cases. Such values are considered adequate for discrimination as revealed in the relevant literature [15, 44].
Conclusions
In conclusion, olive oil alteration due to heating has been detected via LIF at 405 nm. The post-heating fluorescence spectra of adulterated and unadulterated oil samples are suggested to discriminate between different oil samples. After heating to the smoke point, a decrease in the chlorophyll fluorescence peak's intensity was detected in both the unadulterated EVOO (by 62%) and EVOO adulterated with equal ratio of corn or sunflower oil (by 30% and 20% respectively). Partial recovery of the fluorescence peak's intensity has been obtained by unadulterated and corn-adulterated EVOO. The correlation between the obtained repeated measurements were statistically assessed using PLSR method (R2 ~ 0.95 and RMSE ~ 2.056). Additionally, the sensitivity of the proposed method was evaluated using ROC curves giving 88% and 0.93% for EVOO adulterated with corn oil and EVOO adulterated with sunflower oil respectively. The present work provided a simple, portable, sensitive, and rapid method for detecting alteration and adulteration of EVOO.
Data Availability
This manuscript has no associated data.
Code availability
Not applicable.
References
Lazzerini C, Domenici V (2017) Pigments in extra-virgin olive oils produced in tuscany (Italy) in different years. Foods 6. https://doi.org/10.3390/foods.6040.025
Jabeur H, Zribi A, Makni J et al (2014) Detection of chemlali extra-virgin olive oil adulteration mixed with soybean oil, corn oil, and sunflower oil by using GC and HPLC. J Agric Food Chem 62:4893–4904. https://doi.org/10.1021/jf500571n
Al-Ismail KM, Alsaed AK, Ahmad R, Al-Dabbas M (2010) Detection of olive oil adulteration with some plant oils by GLC analysis of sterols using polar column. Food Chem 121:1255–1259. https://doi.org/10.1016/j.foodchem.2010.01.016
Carranco N, Farrés-Cebrián M, Saurina J, Núñez O (2018) Authentication and quantitation of fraud in extra virgin olive oils based on HPLC-UV fingerprinting and multivariate calibration. Foods 7. https://doi.org/10.3390/foods.7040.044
Green HS, Li X, De Pra M et al (2020) A rapid method for the detection of extra virgin olive oil adulteration using UHPLC-CAD profiling of triacylglycerols and PCA. Food Control 107:106773
Meenu M, Cai Q, Xu B (2019) A critical review on analytical techniques to detect adulteration of extra virgin olive oil. Trends Food Sci Technol 91:391–408. https://doi.org/10.1016/j.tifs.2019.07.045
Bilge G, Velioglu Hasan Murat B, Sezer B et al (2016) Identification of meat species by using laser-induced breakdown spectroscopy. Meat Sci 119:118–122. https://doi.org/10.1016/j.meatsci.2016.04.035
Abdel-Salam Z, Abdel-Salam SAM, Harith MA (2017) Application of Laser Spectrochemical Analytical Techniques to Follow Up Spoilage of White Meat in Chicken. Food Anal Methods 10:2365–2372. https://doi.org/10.1007/s12161-017-0806-5
Varnasseri M, Muhamadali H, Xu Y et al (2021) Portable through bottle sors for the authentication of extra virgin olive oil. Appl Sci 11:8347. https://doi.org/10.3390/app11188347
Feng Y, Elmasry G, Sun D et al (2013) Near-infrared hyperspectral imaging and partial least squares regression for rapid and reagentless determination of Enterobacteriaceae on chicken fillets. Food Chem 138:1829–1836. https://doi.org/10.1016/j.foodchem.2012.11.040
Caceres JO, Moncayo S, Rosales JD et al (2013) Application of laser-induced breakdown spectroscopy (LIBS) and neural networks to olive oils analysis. Appl Spectrosc 67:1064–1072. https://doi.org/10.1366/12-06916
Gyftokostas N, Stefas D, Kokkinos V et al (2021) Laser-induced breakdown spectroscopy coupled with machine learning as a tool for olive oil authenticity and geographic discrimination. Sci Rep 11. https://doi.org/10.1038/s41598-021-84941-z
Vanstone N, Moore A, Martos P, Neethirajan S (2018) Detection of the adulteration of extra virgin olive oil by near-infrared spectroscopy and chemometric techniques. Food Qual Saf 2:189–198. https://doi.org/10.1093/fqsafe/fyy018
Gabbarini V, Rossi R, Ciparisse JF et al (2019) Laser-induced fluorescence (LIF) as a smart method for fast environmental virological analyses: validation on Picornaviruses. Sci Rep 9:3–9. https://doi.org/10.1038/s41598-019-49005-3
Hamdy O, Abdel-Salam Z, Abdel-Harith M (2020) Discrimination between fresh, chilled, and frozen/ thawed chicken based on its skin’s spectrochemical and optical properties. Anal Methods 12:2093–2101. https://doi.org/10.1039/d0ay00324g
Hamdy O, Abdel-salam Z, Abdel-harith M (2022) Optical Characterization of Biological Tissues Based on Fluorescence, Absorption, and Scattering Properties. Diagnostics 12:1–14
Alghourani KMK, Bachir W, Karraz G (2020) Effect of Absorption and Scattering on Fluorescence of Buried Tumours. J Spectrosc 8730471. https://doi.org/10.1155/2020/8730471
Peng Y, Dhakal S (2015) Optical Methods and Techniques for Meat Quality Inspection. Trans ASABE 58:1371–1386. https://doi.org/10.13031/trans.58.11004
Lia F, Morote Castellano A, Zammit-Mangion M, Farrugia C (2018) Application of fluorescence spectroscopy and chemometric models for the detection of vegetable oil adulterants in Maltese virgin olive oils. J Food Sci Technol 55:2143–2151. https://doi.org/10.1007/s13197-018-3131-0
Lobo-Prieto A, Tena N, Aparicio-Ruiz R et al (2020) Monitoring virgin olive oil shelf-life by fluorescence spectroscopy and sensory characteristics: A multidimensional study carried out under simulated market conditions. Foods 9:1846. https://doi.org/10.3390/foods9121846
Venturini F, Sperti M, Michelucci U et al (2021) Exploration of spanish olive oil quality with a miniaturized low-cost fluorescence sensor and machine learning techniques. Foods 10:1010. https://doi.org/10.3390/foods10051010
Martín-Tornero E, Fernández A, Pérez-Rodriguez JM et al (2022) Non-destructive Fluorescence Spectroscopy as a Tool for Discriminating Between Olive Oils According to Agronomic Practices and for Assessing Quality Parameters. Food Anal Methods 15:253–265. https://doi.org/10.1007/s12161-021-02112-2
Nikolova K, Zlatanov M, Eftimov T et al (2014) Fluoresence spectra from vegetable oils using violet and blue Ld/Led exitation and an optical fiber spectrometer. Int J Food Prop 17:1211–1223. https://doi.org/10.1080/10942912.2012.700536
Mu T, Chen S, Zhang Y et al (2016) Portable Detection and Quantification of Olive Oil Adulteration by 473-nm Laser-Induced Fluorescence. Food Anal Methods 9:275–279. https://doi.org/10.1007/s12161-015-0199-2
Zhang T, Liu Y, Dai Z et al (2022) Quantitative Detection of Extra Virgin Olive Oil Adulteration, as Opposed to Peanut and Soybean Oil, Employing LED-Induced Fluorescence Spectroscopy. Sensors 22:1–9. https://doi.org/10.3390/s22031227
Abedin KM (2022) Laser-Induced Fluorescence Studies on Some Edible Oils and Aromatic Frankincense Oil Excited by Blue and Violet Diode Lasers at 447 nm and 405 nm. J Spectrosc 2417545
Bavali A, Parvin P, Tavassoli M, Mohebbifar MR (2018) Angular distribution of laser-induced fluorescence emission of active dyes in scattering media. Appl Opt 57:B32–B38. https://doi.org/10.1364/ao.57.000b32
El Orche A, Bouatia M, Mbarki M (2020) Rapid Analytical Method to Characterize the Freshness of Olive Oils Using Fluorescence Spectroscopy and Chemometric Algorithms. J Anal Methods Chem. https://doi.org/10.1155/2020/8860161
Guzmán E, Baeten V, Pierna JAF, García-Mesa JA (2015) Evaluation of the overall quality of olive oil using fluorescence spectroscopy. Food Chem 173:927–934. https://doi.org/10.1016/j.foodchem.2014.10.041
Rinaldi de Alvarenga JF, Quifer-Rada P, Juliano FF et al (2019) Using extra virgin olive oil to cook vegetables enhances polyphenol and carotenoid extractability: A Study Applying the sofrito Technique. Molecules 24:1–17. https://doi.org/10.3390/molecules24081555
Allouche Y, Jiménez A, Gaforio JJ et al (2007) How heating affects extra virgin olive oil quality indexes and chemical composition. J Agric Food Chem 55:9646–9654. https://doi.org/10.1021/jf070628u
Li Y, Chen S, Chen H et al (2020) Effect of thermal oxidation on detection of adulteration at low concentrations in extra virgin olive oil: Study based on laser-induced fluorescence spectroscopy combined with KPCA–LDA. Food Chem 309:125669. https://doi.org/10.1016/j.foodchem.2019.125669
Sikorska E, Khmelinskii I, Sikorski M (2012) Analysis of Olive Oils by Fluorescence Spectroscopy: Methods and Applications. In: Olive Oil - Constituents, Quality, Health Properties and Bioconversions. InTech, pp 63–88
Dartnell LR, Storrie-Lombardi MC, Ward JM (2010) Complete fluorescent fingerprints of extremophilic and photosynthetic microbes. Int J Astrobiol 9:245–257. https://doi.org/10.1017/S1473550410000224
Matveyeva TA, Sarimov RM, Simakin AV et al (2022) Using Fluorescence Spectroscopy to Detect Rot in Fruit and Vegetable Crops. Appl Sci 12:1–11. https://doi.org/10.3390/app12073391
Ergon R (2014) Principal component regression (PCR) and partial least squares regression (PLSR). In: Granato D, Ares G (eds) Mathematical and Statistical Methods in Food Science and Technology. John Wiley & Sons, Ltd
Wentzell PD, Montoto LV (2003) Comparison of principal components regression and partial least squares regression through generic simulations of complex mixtures. Chemom Intell Lab Syst 65:257–279. https://doi.org/10.1016/S0169-7439(02)00138-7
Mehmood T, Liland KH, Snipen L, Sæbø S (2012) A review of variable selection methods in Partial Least Squares Regression. Chemom Intell Lab Syst 118:62–69. https://doi.org/10.1016/j.chemolab.2012.07.010
Bakdash JZ, Marusich LR (2017) Repeated measures correlation. Front Psychol 8:1–13. https://doi.org/10.3389/fpsyg.2017.00456
Pirouz DM (2006) An Overview of Partial Least Squares. Available SSRN 1631359. https://doi.org/10.2139/ssrn.1631359
Abdi H (2010) Partial least squares regression and projection on latent structure regression (PLS Regression). WIREs Comput Stat 2:97–106. https://doi.org/10.1002/wics.51
Arabi DS, Hamdy O, Abdel-Salam ZA et al (2022) Utilization of Spectrochemical Analysis and Diffuse Optical Techniques to Reveal Adulteration of Alike Fish Species and Their Microbial Contamination. Food Anal Methods 15:1062–1073. https://doi.org/10.1007/s12161-021-02212-z
Hamdy O, Mohammed HS (2022) Variations in tissue optical parameters with the incident power of an infrared laser. PLoS One 17:e0263164. https://doi.org/10.1371/journal.pone.0263164
Akobeng AK (2007) Understanding diagnostic tests 3: receiver operating characteristic curves. Acta Pædiatrica 96:644–647. https://doi.org/10.1111/j.1651-2227.2006.00178.x
Nam B-H, D’Agostino RB (2002) Discrimination Index, the Area Under the ROC Curve. Statistics for Industry and Technology. Birkhäuser, Boston, MA, pp 267–279
Zou KH, O’Malley AJ, Mauri L (2007) Receiver-Operating Characteristic Analysis for Evaluating Diagnostic Tests and Predictive Models. Circulation 115:654–657. https://doi.org/10.1161/CIRCULATIONAHA.105.594929
Goncalves L, Subtil A, Oliveira MR, de Bermudez PZ (2014) ROC Curve Estimation : An Overview. REVSTAT – Stat J 12:1–20
Jimenez-Lopez C, Carpena M, Lourenço-Lopes C et al (2020) Bioactive compounds and quality of extra virgin olive oil. Foods 9:1–31. https://doi.org/10.3390/foods9081014
Borello E, Domenici V (2019) Determination of pigments in virgin and extra-virgin olive oils: A comparison between two near UV-vis spectroscopic techniques. Foods 8:4–8. https://doi.org/10.3390/foods8010018
Gorzynik-Debicka M, Przychodzen P, Cappello F et al (2018) Potential health benefits of olive oil and plant polyphenols. Int J Mol Sci 19:1–13. https://doi.org/10.3390/ijms19030686
Bodurov I, Vlaeva I, Marudova M et al (2013) Detection of adulteration in olive oils using optical and thermal methods. Bulg Chem Commun 45:81–85. https://doi.org/10.1063/1.5095358
Zandomeneghi M, Carbonaro L, Caffarata C (2005) Fluorescence of vegetable oils: Olive oils. J Agric Food Chem 53:759–766. https://doi.org/10.1021/jf048742p
Alzaa DF, Guillaume C, Ravetti L (2018) Evaluation of Chemical and Physical Changes in Different Commercial Oils during Heating. Acta Sci Nurtitional Heal 2:2–11
Cheikhousman R, Zude M, Bouveresse DJR et al (2004) Fluorescence Spectroscopy for Monitoring Extra Virgin Olive Oil Deterioration Upon Heating. Czech J Food Sci 22:147–150. https://doi.org/10.1007/s00216-005-3286-1
Rasul HH, Inanc AL (2014) Thermal Stability of Chlorophyll Pigments in Virgin Olive Oil. KSU J Nat Sci 17:34–40
Balaky HH, Rasul NH, Khudher HA et al (2020) Effect of Heating on Changes of Chlorophyll Content and Oxidative Stability in Olive Pomace Oil. J Crit Rev 7:8282–8287. https://doi.org/10.31838/jcr.07.19.935
Saleem M, Ahmad N, Ali H et al (2017) Investigating temperature effects on extra virgin olive oil using fluorescence spectroscopy. Laser Phys 27:1–10. https://doi.org/10.1088/1555-6611/aa8cd7
Mahmood Z, Khan S (2009) On the Use of K-Fold Cross-Validation to Choose Cutoff Values and Assess the Performance of Predictive Models in Stepwise Regression. Int J Biostat 5:25. https://doi.org/10.2202/1557-4679.1105
Wang H, Wang K, Zhu X et al (2020) Integration of Partial Least Squares Regression and Hyperspectral Data Processing for the Nondestructive Detection of the Scaling Rate of Carp (Cyprinus carpio). Foods 9:1–18. https://doi.org/10.3390/foods9040500
Acknowledgement
The authors would like to thank Prof. Mohamed Abdel-Harith and Prof. Zienab Abdel-Salam (Laser Applications in Metrology, Photochemistry and Agriculture Dept., NILES, Cairo University) for their help in evaluating the chlorophyll-a contents of the EVOO samples.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
Conceptualization: Haitham S. Mohammed. Data curation: Omnia Hamdy, Haitham S. Mohammed. Formal analysis: Omnia Hamdy, Haitham S. Mohammed. Investigation: Omnia Hamdy. Methodology: Omnia Hamdy. Supervision: Haitham S. Mohammed. Validation: Omnia Hamdy. Visualization: Omnia Hamdy. Writing original draft: Omnia Hamdy. Writing, review & editing: Haitham S. Mohammed.
Corresponding author
Ethics declarations
Ethical Approval
No ethical approval is required for this study.
Consent to participate
Not applicable.
Consent for publication
All authors have read and approved the final manuscript.
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hamdy, O., Mohammed, H.S. Post-heating Fluorescence-based Alteration and Adulteration Detection of Extra Virgin Olive Oil. J Fluoresc 33, 1631–1639 (2023). https://doi.org/10.1007/s10895-023-03165-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-023-03165-8