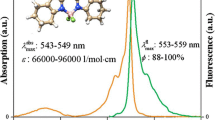
Abstract
Dipyrrolydiketones BF2 complex was synthesized and characterized by NMR, HRMS, and single crystal diffraction. In non-polar environment, this BF2 containing dye emitted bright blue-green fluorescence. No significant spectra shift was observed both in absorption and emission spectra, which indicates the insensitivity of absorption/emission toward environment. The alkyl substituted pyrrole rings lead to its highly emission character in solid state by enhancing the distance between dye molecules. Absolute quantum yields were determined to be 0.51–0.78/0.36 in selected organic medium and solid state, respectively. The emission dynamics was investigated by fluorescence lifetime and both monoexponential and bi-exponential decay was observed.








Similar content being viewed by others
References
Cao D, Liu Z, Verwilst P, Koo S, Jangjili P, Kim JS, Lin W (2019) Coumarin-based small-molecule fluorescent chemosensors. Chem Rev 119:10403–10519. https://doi.org/10.1021/acs.chemrev.9b00145
Mutoh K, Miyashita N, Arai K, Abe J (2019) Turn-on mode fluorescence switch by using negative photochromic imidazole dimer. J Am Chem Soc 141:5650–5654. https://doi.org/10.1021/jacs.9b01870
Kobayashi Y, Abe J (2016) Real-time dynamic hologram of a 3D object with fast photochromic molecules. Adv Optical Mater 4:1354–1357. https://doi.org/10.1002/adom.201600218
Tian H (2010) Data processing on a unimolecular platform. Angew Chem Int Ed 49:4710–4712. https://doi.org/10.1002/anie.200906834
Jin P, Jiao C, Guo Z, He Y, Zhu S, Tian H, Zhu W (2014) Rational design of a turn-on fluorescent sensor for α-ketoglutaric acid in a microfluidic chip. Chem Sci 5:4012–4016. https://doi.org/10.1039/C4SC01378F
Wasielewski MR (1992) Photoinduced electron transfer in supramolecular systems for artificial photosynthesis. Chem Rev 92:435–461. https://doi.org/10.1021/cr00011a005
Li X, Kim SH, Son YA (2009) Optical properties of donor-π-(acceptor)n merocyanine dyes with dicyanovinylindane as acceptor group and triphenylamine as donor unit. Dyes Pigm 82:293–298. https://doi.org/10.1016/j.dyepig.2008.12.014
Li X, Zhou Q, Heo G, Son YA (2018) 2,4-Dimethylpyrrole configured fluorine-boron complexes. Mol Cryst Liq Cryst 677:34–41. https://doi.org/10.1080/15421406.2019.1597509
Mao J, Wang L, Dou W, Tang X, Yan Y, Liu W (2007) Tuning the selectivity of two chemosensors to Fe(III) and Cr(III). Org Lett 9:4567–4570. https://doi.org/10.1021/ol7020687
Zhang X, Yasuhir S, Takayuki H (2007) Cu(II)-Selective Green fluorescence of a rhodamine−diacetic acid conjugate. Org Lett 9:5039–5042. https://doi.org/10.1021/ol7022714
Li X, Han Y, Min K, Son YA (2018) Configuration of white light emission by courmarin and naphthalimide. Mol Cryst Liq Cryst 660:10–16. https://doi.org/10.1080/15421406.2018.1452861
Li X, Shan D, Son YA (2016) High pseduo-stoke’s shift of a naphthalene-bisindolylmaleimide dye. J Nanosci Nanotechnol 16:856–860. https://doi.org/10.1166/jnn.2016.11779
Sheng Y, Ma J, Liu S, Wang ZhuC, Cheng Y (2016) Strong and reversible circularly polarized luminescence emission of a chiral 1,8-naphthalimide fluorophore induced by excimer emission and orderly aggregation. Chem Eur J 22:9519–9522. https://doi.org/10.1002/chem.201600891
Ulrich G, Ziessel R, Harriman A (2008) The chemistry of fluorescent bodipy dyes: Versatility unsurpassed. Angew Chem Int Ed 47:1184–1201. https://doi.org/10.1002/anie.200702070
Li X, Han Y, Kim MJ, Son YA (2018) A BODIPY-based highly emissive dye with thiophene-based branch harvesting the light. Mol Cryst Liq Cryst 662:157–164. https://doi.org/10.1080/15421406.2018.1467613
Yoshii R, Yamane H, Nagai A, Tanaka K, Taka H, Kita H, Chujo Y (2014) π-Conjugated polymers composed of BODIPY or aza-BODIPY derivatives exhibiting high electron mobility and low threshold voltage in electron-only devices. Macromolecules 47:2316–2323. https://doi.org/10.1021/ma5002047
Rousseau T, Cravino A, Bura T, Ulrich G, Ziessel R, Roncali J (2009) BODIPY derivatives as donor materials for bulk heterojunction solar cells. Chem Commun 13:1673–1675. https://doi.org/10.1039/B822770E
Olivier JH, Camerel F, Ulrich G, Barberá J, Ziessel R (2010) Luminescent ionic liquid crystals from self-assembled BODIPY disulfonate and imidazolium frameworks. Chem Eur J 16:7134–7142. https://doi.org/10.1002/chem.201000339
Jokic T, Borisov SM, Saf R, Nielsen DA, Kühl M, Klimant I (2012) Highly photostable near-Infrared fluorescent pH indicators and sensors based on BF2-chelated tetraarylazadipyrromethene dyes. Anal Chem 84:6723–6730. https://doi.org/10.1021/ac3011796
Aguiar A, Farinhas J, da Silva W, Susano M, Silva MR, Alcacer L, Kumar S, Brett CMA, Morgado J, Sobral AJFN (2020) Simple BODIPY dyes as suitable electron-donors for organic bulk heterojunction photovoltaic cells. Dyes Pigm 172:107842. https://doi.org/10.1016/j.dyepig.2019.107842
Li X, Han Y, Sun S, Shan D, Ma X, He G, Mergu N, Park JS, Kim CH, Son YA (2020) A diaminomaleonitrile-appended BODIPY chemosensor for the selective detection of Cu2+ via oxidative cyclization and imaging in SiHa cells and zebrafish. Spectrochim Acta A 233:118179. https://doi.org/10.1016/j.saa.2020.118179
Zh H, Fan J, Wang J, Mu H, Peng X (2014) An enhanced PET-based fluorescent probe with ultrasensitivity for imaging basal and elesclomol-induced HClO in cancer cells. J Am Chem Soc 136:12820–12823. https://doi.org/10.1021/ja505988g
Devaraj NK, Hilderbrand S, Upadhyay R, Mazitschek R, Weissleder R (2010) Bioorthogonal turn-on probes for imaging small molecules inside living cells. Angew Chem Int Ed 49:2869–2872. https://doi.org/10.1002/anie.200906120
Boens N, Verbelen B, Ortiz MJ, Jiao LJ, Dehaen W (2019) Synthesis of BODIPY dyes through postfunctionalization of the boron dipyrromethene core. Coord Chem Rev 399:213024. https://doi.org/10.1016/j.ccr.2019.213024
Kamkaew A, Lim SH, Lee HB, Kiew LV, Chung LY, Burgess K (2013) BODIPY dyes in photodynamic therapy. Chem Soc Rev 42:77–88. https://doi.org/10.1039/C2CS35216H
Hong Y, Lam JWY, Tang BZ (2011) Aggregation-induced emission. Chem Soc Rev 40:5361–5388. https://doi.org/10.1039/C1CS15113D
Chen W, Chen CL, Zhang Z, Chen YA, Chao WC, Su J, Tian H, Chou PT (2017) Snapshotting the excited-state planarization of chemically locked N, N′-disubstituted dihydrodibenzo[a, c]phenazines. J Am Chem Soc 139:1636–1644. https://doi.org/10.1021/jacs.6b11789
Zhang Z, Wu YS, Tang KC, Chen CL, Ho JW, Su J, Tian H, Chou PT (2015) Excited-state conformational/electronic responses of saddle-shaped N, N′-disubstituted-dihydrodibenzo[a, c]phenazines: Wide-tuning emission from red to deep blue and white light combination. J Am Chem Soc 137:8509–8520. https://doi.org/10.1021/jacs.5b03491
Massue J, Frath D, Ulrich G, Retailleau P, Ziessel R (2012) Synthesis of luminescent 2-(2′-hydroxyphenyl)benzoxazole (HBO) borate complexes. Org Lett 14:230–233. https://doi.org/10.1021/ol203014e
Frath D, Massue J, Ulrich G, Ziessel R (2014) Luminescent materials: Locking π-conjugated and heterocyclic ligands with boron(III). Angew Chem Int Ed 53:2290–2310. https://doi.org/10.1002/anie.201305554
Liao CW, Rajeswara RM, Sun SS (2015) Structural diversity of new solid-state luminophores based on quinoxaline-β-ketoiminate boron difluoride complexes with remarkable fluorescence switching properties. Chem Commun 51:2656–2659. https://doi.org/10.1039/C4CC08958H
Li X, Han Y, Kim M, Son YA (2017) Absorption and emission investigation of boroncored dye. Mol Cryst Liq Cryst 659:64–70. https://doi.org/10.1080/15421406.2018.1450926
Li X, Son YA (2014) Efficient luminescence from easily prepared fluorine–boron core complexes based on benzothiazole and benzoxazole. Dyes Pigm 107:182–187. https://doi.org/10.1016/j.dyepig.2014.04.001
Delley B (1990) An all-electron numerical method for solving the local density functional for polyatomic molecules. J Chem Phys 92:508–517. https://doi.org/10.1063/1.458452
Delley B (2000) From molecules to solids with the DMol3 approach. J Chem Phys 113:7756–7764. https://doi.org/10.1063/1.1316015
Funding
This work was supported by the National Natural Science Foundation of China (grant no. 21772034) and the Program for Innovative Research Team in Science and Technology in Universities of Henan Province (grant No. 19IRTSTHN023, 20IRTSTHN005). We also thank the financial support from Henan Key Laboratory of Organic Functional Molecules and Drug Innovation.
Author information
Authors and Affiliations
Contributions
All the authors (Xinyu Guo, Yunfneg Chen, Ting Cui, Lina Xing, and Xiaochuan Li) made substantial contribution while preparing the manuscript.
Corresponding author
Ethics declarations
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare that they have no competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Li, X., Guo, X., Chen, Y. et al. Double 3-Ethyl-2,4-dimethylpyrrole Configured Fluorescent Dye with Fluorine-Boron as the Bridge. J Fluoresc 31, 1797–1803 (2021). https://doi.org/10.1007/s10895-021-02819-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-021-02819-9